Researchers develop microfluidic chip to stem flu outbreaks

The H1N1 flu pandemic in 2009 underscored weaknesses in methods widely used to diagnose the flu, from frequent false negatives to long wait times for results. Now Boston University researchers have developed a prototype of a rapid, low-cost, accurate, point-of-care device that promises to provide clinicians with an effective tool to quickly diagnose both seasonal and pandemic strains of influenza, and thus limit the spread of infection.
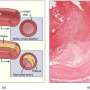
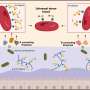
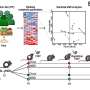
Boston University Biomedical Engineering Associate Professor Catherine Klapperich led the team of engineering and medical researchers that developed the disposable microfluidic chip the size of a microscope slide that aims to take the place of expensive, time-consuming, laboratory-based diagnostic tests now in use. The chip essentially miniaturizes the RT-PCR test, now considered the gold standard in flu detection, producing faster, cheaper and accurate results.
The researchers collected clinical nasal specimens and placed them on the chip, which extracts RNA from the Influenza A virus, converts it into DNA and replicates the sample in sufficient quantities so it can be detected by a table-top external reader. The chip produced results that matched the accuracy of the lab-based tests.
"We wanted to show that our technique was feasible on real-world samples prepared on the chip," said Klapperich. "Making each chip single-use decreases the possibility of cross-contamination between specimens, and the chip's small size makes it a good candidate for true point-of-care testing."
The microfluidic chip also proved far more effective than other commonly used flu diagnostic tests including viral culture, a lab procedure requiring up to a week to produce results; rapid immunoassays, which work like pregnancy tests but were only 40 percent reliable in detecting the presence of a flu virus in this study; and direct fluorescent antigen testing (DFA), a more accurate but labor-intensive process in which medical personnel prepare and interpret samples stained with fluorescent antibodies.
"The new test represents a major improvement over viral culture in terms of turn-around time, over rapid immunoassay tests in terms of sensitivity (ability to detect the virus from minimal sample material) and over DFA and RT-PCR in terms of ease of use and portability," Klapperich observed.
Ultimately seeking to enable clinicians to use their microfluidic chips for frontline flu virus detection, the researchers next plan to optimize their method so that it can produce results in a third less time (an hour) with chips that cost half as much to make (five dollars). In addition, they are exploring ways to develop a lower cost external reader that's no bigger than a clinical digital thermometer.
More information: The results were published in the March 22 online edition of PLoS ONE.