Clues found to way embryonic kidney maintains its fleeting stem cells
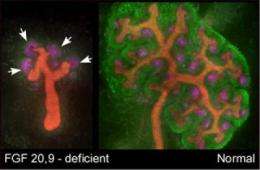
Studying mice and humans, researchers at Washington University School of Medicine in St. Louis and their collaborators in Paris have identified two proteins that are required to maintain a supply of stem cells in the developing kidney.
In the presence of the two proteins, FGF9 and FGF20, mouse kidney stem cells stayed alive outside the body longer than previously reported. Though the cells were maintained only five days (up from about two), the work is a small step toward the future goal of growing kidney stem cells in the lab.
In the developing embryo, these early stem cells give rise to adult cells called nephrons, the blood filtration units of the kidneys.
The results appear online June 11 in Developmental Cell.
"When we are born, we get a certain allotment of nephrons," says Raphael Kopan, PhD, the Alan A. and Edith L. Wolff Professor of Developmental Biology. "Fortunately, we have a large surplus. We can donate a kidney — give away 50 percent of our nephrons — and still do fine. But, unlike our skin and gut, our kidneys can't build new nephrons."
The skin and the gut have small pools of stem cells that continually renew these organs throughout life. Scientists call such pools of stem cells and their support system a niche. During early development, the embryonic kidney has a stem cell niche as well. But at some point before birth or shortly after, all stem cells in the kidney differentiate to form nephrons, leaving no self-renewing pool of stem cells.
"In other organs, there are cells that specifically form the niche, supporting the stem cells in a protected environment," Kopan says. "But in the embryonic kidney, it seems the stem cells form their own niche, making it a bit more fragile. And the signals and conditions that lead the cells to form this niche have been elusive."
Surprisingly, recent clues to the signals that maintain the embryonic kidney's stem cell niche came from studies of the inner ear. David M. Ornitz, MD, PhD, the Alumni Endowed Professor of Developmental Biology, investigates FGF signaling in mice. Earlier this year, Ornitz and his colleagues published a paper in PLoS Biology showing that FGF20 plays an important role in inner ear development.
"Mice without FGF20 are profoundly deaf," Ornitz says. "While they are otherwise viable and healthy, in some cases we noticed that their kidneys looked small."
Past work from his own lab and others suggested that FGF9, a close chemical cousin of FGF20, might also participate in kidney development. FGF20 and FGF9 are members of a family of proteins known as fibroblast growth factors. In general, members of this family are known to play important and broad roles in embryonic development, tissue maintenance, and wound healing. Mice lacking FGF9 have defects in development of the male urogenital tract and die after birth due to defects in lung development.
Since FGF9 and 20 are so closely related, Ornitz and Sung-Ho Huh, PhD, a postdoctoral research associate, and former postdoctoral researcher Hila Barak, PhD, thought the two proteins might serve redundant functions in the developing kidney, compensating for each other if one is missing. The next logical question, according to Ornitz, was what happens when both are missing.
"When we examined mice lacking both FGF9 and FGF20, we saw that the embryos had no kidneys," Ornitz says.
Evidence further supporting the role of FGF signaling in kidney development came from collaborators in France. There, researchers had identified two families that lost more than one pregnancy because the fetuses developed without kidneys, a condition called renal agenesis.
Analyzing DNA from the fetuses, they found mutations in four genes known to be involved in kidney development. One of these mutations resulted in the total loss of FGF20. This analysis also highlighted an important difference between mice and humans. In mice, FGF9 appears to compensate for a total loss of FGF20. But in the human DNA analysis, the fetuses that developed without kidneys had normal FGF9.
"In humans, FGF9 is not enough to rescue the kidney if FGF20 is missing," Kopan says.
Now that at least some of the proteins necessary to support the kidney stem cell niche have been identified, Kopan and Ornitz both look to future work to further extend the life of such cells in the lab.
"The holy grail would be to deliver these cells back to a diseased kidney," Kopan says. "This is a very small step. But we hope this will be a stimulus to the field, for us and for others to continue thinking about how to convince these cells to stick around longer."
More information: Barak H, Huh SH, Chen S, Jeanpierre C, Martinovic J, Parisot M, Bole-Feysot C, Nitschke P, Salomon R, Antignac C, Ornitz DM, Kopan R. FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Developmental Cell. Online June 11, 2012.
Huh SH, Jones J, Warchol ME, Ornitz DM. Differentiation of the lateral compartment of the cochlea requires a temporally restricted FGF20 signal. PLoS Biology. Jan. 3, 2012.















