New gene transfer strategy shows promise for limb girdle and other muscular dystrophies
The challenge of treating patients with genetic disorders in which a single mutated gene is simply too large to be replaced using traditional gene therapy techniques may soon be a thing of the past. A Nationwide Children's Hospital study describes a new gene therapy approach capable of delivering full-length versions of large genes and improving skeletal muscle function. The strategy may hold new hope for treating dysferlinopathies and other muscular dystrophies.
A group of untreatable muscle disorders known as dysferlinopathies are caused by mutations in the dysferlin gene. Patients with these disorders, including limb girdle muscular dystrophy type 2B, are typically diagnosed in their early twenties. Approximately one-third will become wheelchair dependent by their mid-30s.
Gene therapy using adeno-associated virus (AAV) to deliver genes to cells has been pursued as an option for some patients with muscular dystrophy. However, AAV's packaging limitations have served as obstacles in using gene therapy to deliver large genes like dysferlin. Scientists in the past have attempted to work around AAV's packaging limitations by inserting a small version of large genes into the viral vector to induce gene expression. Some have also used more than one viral vector at a time to deliver a large gene. However, micro and mini versions of large genes don't always have the power of full-length gene expression and an increased viral load can lead to negative side effects.
"We have had success in the clinic using AAV gene therapy with limb girdle muscular dystrophy type 2D, which is caused by mutations in the alpha-sarcoglycan gene," said Louise Rodino-Klapac, PhD, principal investigator in the Center for Gene Therapy at The Research Institute of Nationwide Children's Hospital. "However, the dysferlin gene is very large, about six times larger than the alpha-sarcoglycan gene and can't fit into a traditional AAV vector."
A 2008 study identified AAV5, an AAV serotype that could package large transcripts. "This made us wonder whether it could be used for gene replacement requiring inserts as large as the dysferlin gene," said Dr. Rodino-Klapac.
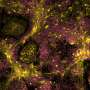
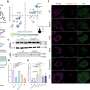
In their 2012 study appearing in PLoS ONE, Dr. Rodino-Klapac's team used AAV5 to package a full-length, intact dysferlin gene and directly deliver it to the diaphragm of dysferlin-deficient mice. They also injected the leg muscles of dysferlin-deficient mice using both intramuscular and vascular approaches to further evaluate whether the gene delivery could improve skeletal muscle function.
They found that both the intravascular and intramuscular delivery approaches led to full-length, intact dysferlin gene expression in the leg and diaphragm muscle cells of the mice. More importantly, they saw that the newly-restored dysferlin repaired membrane deficits previously seen in the dysferlin-deficient mice.
"Our findings demonstrate highly favorable results with full restoration of dysferlin without compromise in function," said Dr. Rodino-Klapac. "With regard to neuromuscular diseases, these studies provide new perspective for conditions caused by mutations of large genes. Duchenne muscular dystrophy is the most common severe childhood muscular dystrophy and would seem to benefit from expression of the larger transcripts than mini- and micro-dystrophins that only partially restore physiologic function in mouse models of the disease."
Dr. Rodino-Klapac and her team are currently defining a path for a dysferlin clinical gene therapy trial. "We have shown that AAV5-dysferlin delivery is a very promising therapeutic approach that could restore functional deficits in dysferlinopathy patients," she says.
More information: Grose WE, Clark KR, Griffin D, Malik V, Shontz KM, Montgomery CL, Lewis S, Brown RH Jr, Janssen PM, Mendell JR, Rodino-Klapac LR. Homologous Recombination Mediates Functional Recovery of Dysferlin Deficiency following AAV5 Gene Transfer. PLoS One. 2012;7(6):e39233. Epub 2012 Jun 15.