A drug-screening platform for ALS
A research group at the Center for iPS Cell Research and Application (CiRA) at Japan's Kyoto University has successfully recapitulated amyotrophic lateral sclerosis (ALS)-associated abnormalities in motor neurons differentiated from induced pluripotent stem cells (iPSCs) obtained from patients with familial ALS, a late-onset, fatal disorder which is also known for Lou Gehrig's disease. In a drug screening assay using the disease model, the team further found that the chemical compound anacardic acid can rescue some ALS phenotypes in vitro.
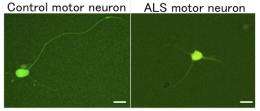
In a study published online in Science Translational Medicine, Associate Professor Haruhisa Inoue and his team generated motor neurons from iPSCs derived from three ALS patients with mutations in Tar DNA-binding protein-43 (TDP-43). The motor neurons showed cellular phenotypes including vulnerability to stress, shorter neurites, and cytosolic aggregates similar to those seen in postmortem tissues from ALS patients. The team also found that TDP-43 mRNA was upregulated in the ALS motor neurons, which means that TDP-43 autoregulation was disturbed, and that TDP-43 protein in detergent-insoluble form aggregated with the splicing factor SNRPB2 in the nucleus, perturbing RNA metabolism. These findings shed light on the mechanism of disease onset.
Using the motor neurons as a disease model, the researchers discovered that the chemical compound anacardic acid can rescue the abnormal ALS motor neuron phenotypes. For example, when anacardic acid, a histone acetyltransferase inhibitor, was sprinkled on the motor neurons, TDP-43 mRNA expression was decreased, and the length of the neurites increased.
''Our work represents an initial stage of drug screening for ALS using patient-specific iPSCs. TDP-43 is not only relevant to familial ALS but also to sporadic ALS, which represents the majority of ALS cases,'' said Inoue, a principal investigator at CiRA who is also one of research directors for the CREST research program funded by the Japan Science and Technology Agency. ''We will continue to work on ALS patient-specific iPSCs in order to help develop new drug seeds and candidates.''
More information: Egawa et al. "Drug Screening for ALS Using Patient-Specific Induced Pluripotent Stem Cells" Science Translational Medicine.