Understanding abnormal proteins in degenerative diseases
Amyloids, or fibrous aggregates of abnormally folded proteins, are a common feature in degenerative diseases such as Alzheimer's, diabetes and cancer. Amyloids occur naturally in the body, but despite decades of research, their mechanism of formation remains unknown, hampering drug development efforts. Now, a new class of ultrasmall peptides developed by the Institute of Bioengineering and Nanotechnology (IBN) offers scientists a platform for understanding this phenomenon, providing them with the insights required to design more effective treatments for these diseases.
IBN Executive Director Professor Jackie Y. Ying said, "Our researchers have been focusing on creating biomimetic materials for nanomedicine and cell and tissue engineering applications. The novel ultrasmall peptides developed by IBN are not only highly effective as synthetic cell culture substrates, but also as a model for studying the mystery of amyloid formation. Such fundamental understanding could contribute towards advancing medical treatment of amyloid-related disorders."
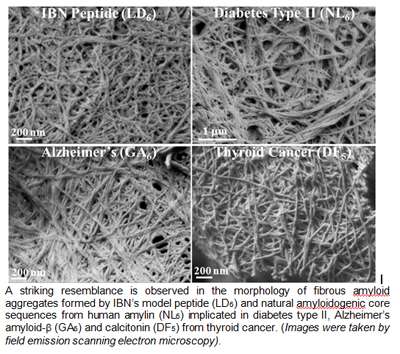
First discovered in 2011 by IBN Team Leader and Principal Research Scientist Dr Charlotte Hauser, the peptides were formed from only 3-7 amino acids, making them the smallest ever reported class of self-assembling aliphatic compounds. Peptides perform a wide range of functions in the body, and are distinguished from proteins based on size. Building on this earlier research, IBN researchers have found a striking similarity between the structure of their synthetic peptides and the protein structure of naturally occurring amyloids in the latest study published in Proceedings of the National Academy of Sciences.
Dr Hauser elaborated, "This is the first proof-of-concept that our peptides self-assemble in the same way as naturally occurring amyloid sequences. Knowing that the process of amyloid formation is common across various chronic degenerative diseases, our goal is to identify the specific trigger so that we can design the appropriate drugs to inhibit and control the aggregate formation."
The IBN team collaborated with researchers from the Institute of High Performance Computing and the European Synchrotron Radiation Facility to validate their peptides with the core protein sequences of three diseases: Alzheimer's, diabetes and thyroid cancer.
The results revealed that the mechanism behind the self-assembly of amyloids from smaller intermediate structures into larger amyloid structures was similar to how the IBN peptides were formed. In addition, this study supports the growing evidence that early intermediates are more toxic than the final amyloid fibers, and may even be the driving force behind amyloid formation.
Patent applications have been filed on this research, and the next step of this project is pre-clinical evaluation of ultrasmall peptide therapeutics. IBN will also investigate other amyloid disorders such as corneal dystrophy, which can result in blindness.
What are peptides?
Peptides are short to medium length sequences of amino acids, differentiated from proteins based on their smaller size. In proteins, the molecules fold into a specific three-dimensional structure that influences its activity or function in the body. Amyloids comprise distinctly structured aggregates that are formed by functional, but also abnormally folded proteins in the body.
More information: References:
1. A. Lakshmanan, D. W. Cheong, A. Accardo, E. Di Fabrizio, C. Riekel and C. A. E. Hauser, "Aliphatic Peptides Show Similar Self-Assembly to Amyloid Core Sequences, Challenging the Importance of Aromatic Interactions in Amyloidosis," Proceedings of the National Academy of Sciences 110 (2013) 519-524.,
2. C. A. E. Hauser, R. Deng, A. Mishra, Y. Loo, U. Khoe, F. Zhuang, D. W. Cheong, A. Accardo, M. B. Sullivan, C. Riekel, J. Y. Ying and U. A. Hauser, "Natural Tri- to Hexapeptides Self-Assemble in Water to Amyloid β-Type Fibre Aggregates by Unexpected α-Helical Intermediate Structures," Proceedings of the National Academy of Sciences, 108 (2011) 1361-1366.