When cells are consumed by wanderlust
(Medical Xpress)—In experiments on zebrafish, Freiburg researchers have demonstrated that the same proteins that lead to the formation of metastases in humans also cause the cells to migrate during embryonic development. The study was conducted by a team headed by Prof. Dr. Wolfgang Driever and Prof. Dr. Thomas Brabletz and including researchers from the Department of Developmental Biology, the Department of Visceral Surgery at the University Medical Center, and the Cluster of Excellence BIOSS Centre for Biological Signalling Studies. The scientists hope their findings on cell migration in zebrafish will open up new perspectives for research on proteins that control metastasis and thus the malignancy of cancer.
Cells are usually firmly connected to one another in their tissues. In order to move through the tissue, they must break away and modify their contacts with other cells, which are made by way of binding proteins. This happens particularly often during the stage of embryonic development, but it can also occur in tumors when they form metastases. In cancer cells, the protein networks that control the behavior are changed in such a way as to cause the cells to lose control and break away - a mutated skin cell, for instance, might then migrate into the bloodstream. This behavior has long been described, but there are still many open questions on the control mechanisms regulating this migration. In the Journal of Biological Chemistry, the scientists demonstrate that the protein ZEB1 plays a particularly important role: It limits the production of the binding proteins E-cadherin and Epcam - both in the fish embryo and in the tumor cell. The less E-cadherin and Epcam are produced, the looser the bonds to other cells become.
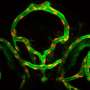
The cell migrations are particularly easy to observe in the zebrafish, because the embryo is transparent. The phase of gastrulation, in which the germ layers form through invagination of the outermost cell layer, is the first great cell migration phase in the development of vertebrates. The cells migrate from the surface of the embryo to their target position inside of the organism. The zebrafish has two genes resembling the ZEB1 gene that are involved in gastrulation: zeb1a and zeb1b. The researchers switched these genes on and off and followed the development of the modified fish embryos under the microscope in order to determine the function of the protein ZEB1.
Their observations show that embryos that possess too much or too little of the protein ZEB1 do not develop normally because they cannot execute the gastrulation movements correctly. ZEB1 checks the formation of the binding proteins E-cadherin and Epcam, enabling cells to leave their cell networks - exactly like tumor cells during metastasis in humans. The findings of the project also reveal how the same mechanisms of cell migration remain intact in the course of evolution, making it possible to draw comparisons between fish and humans.