This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Knocking out deadly brain cancer with a dual therapy
Glioblastoma is an often fatal form of brain cancer, with only 5% of patients surviving beyond five years. The cancer is difficult to treat and almost always becomes resistant to treatment. As a result, recurrence of glioblastoma is practically inevitable.
Findings from a joint study by researchers from NTU's Lee Kong Chian School of Medicine (LKCMedicine) and the National Neuroscience Institute (NNI) offer new hope for glioblastoma patients. Their work could pave the way for more effective and precise therapies for the deadly disease and prevent the cancer from recurring.
The research, "Dual p38MAPK and MEK inhibition disrupts adaptive chemoresistance in mesenchymal glioblastoma to temozolomide," has been published in Neuro-Oncology.
Currently, a chemotherapy drug called temozolomide (TMZ) is used to treat glioblastoma. TMZ damages the DNA of cancer cells, preventing the cells from dividing. However, glioblastoma almost always grows back as the cancer eventually develops resistance to TMZ.
Resistance develops because glioblastoma tumors consist of populations of cells with different properties—a characteristic known as genetic heterogeneity—making it more likely that some cells will adapt to and resist the treatment.
There is also a dearth of treatment options for resistant glioblastoma, as not many drugs can cross the blood-brain barrier, a protective membrane that controls the passage of substances into the brain.
Delivering a double blow to brain tumors
To understand the cellular mechanism behind drug resistance and find potential drug targets for resistant glioblastoma, the researchers compared the activity of protein kinases—enzymes involved in cellular signaling pathways associated with cancer growth and spread—in mesenchymal glioblastoma (ME) and proneural glioblastoma (PN) cells derived from patients.
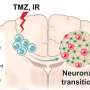
ME is the most aggressive type of glioblastoma that is resistant to treatment, while patients with PN have more favorable outcomes. During treatment, PN glioblastoma can transform into ME glioblastoma, leading to cancer relapses.
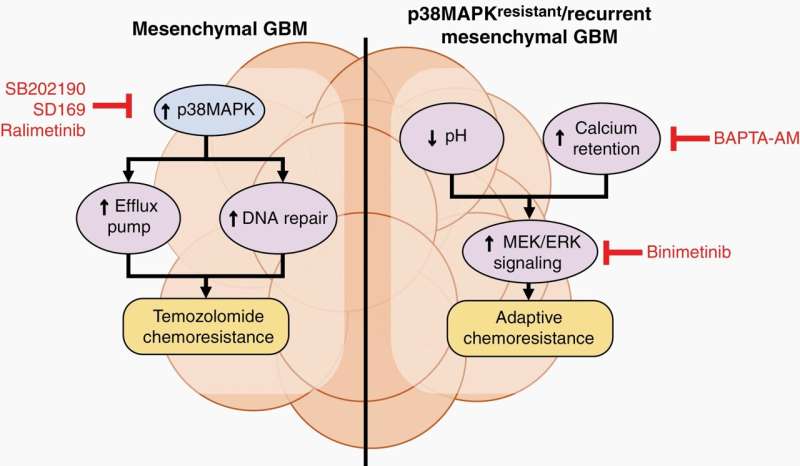
The scientists found that compared to PN, a type of protein kinase called mitogen-activated protein kinases (MAPK) was activated in ME. In particular, the activities of two MAPK, p38MAPK and MEK/ERK, were upregulated.
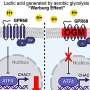
In ME, p38MAPK signaling increases the activity of transporter proteins that pump out drugs from cells. The p38MAPK signaling pathway also improves the ability of glioblastoma cells to repair DNA damage caused by TMZ. These processes enable the cancer cells to survive treatment and contribute to the innate ability of ME to resist treatment.
On the other hand, MEK/ERK signaling pathways are activated when glioblastoma develops resistance following drug treatment—a phenomenon known as adaptive resistance. The researchers observed that when p38MAPK is inhibited, pH inside the cells decreases and calcium increases, which triggers MEK/ERK signaling and increases the survival of glioblastoma cells. The drop in pH also affects the conversion of TMZ to its active compound, reducing its effectiveness.
Repurposing drugs for personalized cancer treatment
The researchers implanted ME cells from patients into mice. They found that mice treated with a combination of p38MAPK inhibitor ralimetinib, MEK inhibitor binimetinib, and TMZ had the best survival of 72.5 days compared to mice treated with TMZ alone (63 days).
Binimetinib, also known as Mektovi, has been approved by the FDA to treat melanoma, while ralimetinib has been tested in a Phase I trial to treat glioblastoma.
Ralimetinib and binimetinib inhibited p38MAPK and MEK/ERK, respectively, restoring the effectiveness of TMZ against ME. Inhibiting p38MAPK also decreased the expression of various drug transporter proteins and enhanced the retention of TMZ in cells.
Assoc Prof Andrew Tan of LKCMedicine, who co-led the research, said "Our study has shown that glioblastoma acquires drug resistance through multiple pathways, highlighting the need for more precise treatments of the disease."
"Instead of using a single drug, therapies that target the innate and adaptive mechanisms of drug resistance simultaneously could be feasible treatments for resistant glioblastoma tumors," said Dean's Postdoctoral Fellow at LKCMedicine Dr. Hong Sheng Cheng, who is the first author of the study.
"We have demonstrated that the repurposing of existing drugs is a key strategy to maximize the implementation of precision medicine in cancer, especially when treating highly recurrent tumors like glioblastoma," said Assoc Prof Ang Beng Ti, senior consultant at the Department of Neurosurgery and co-Principal Investigator at the Neuro-Oncology Lab in NNI, one of the co-lead investigators.
Commenting as an independent expert, Prof Pierce Chow, a clinician-scientist who leads research in liver cancer and precision medicine for cancer treatment, said, "Drug resistance is one of the most challenging aspects of cancer treatment. I congratulate the NNI and NTU team on leveraging established knowledge about drug properties to reposition existing drugs. Their work points to a safer and more effective way to develop personalized therapies for resistant cancers."
Prof Chow is senior consultant surgeon at the National Cancer Center Singapore and the Singapore General Hospital and Professor at the Duke-NUS Medical School.
The researchers plan to conduct clinical trials to bring the treatment one step closer to the clinic. They also intend to employ cutting-edge molecular profiling techniques alongside artificial intelligence technologies, such as machine learning, to refine the strategic combination and delivery of drugs for treating glioblastoma.
More information: Hong Sheng Cheng et al, Dual p38MAPK and MEK inhibition disrupts adaptive chemoresistance in mesenchymal glioblastoma to temozolomide, Neuro-Oncology (2024). DOI: 10.1093/neuonc/noae028