July 24, 2013 feature
Splice this: End-to-end annealing demonstrated in neuronal neurofilaments
While popularly publicized neuroscience research focuses on structural and functional connectomes, timing patterns of axonal spikes, neural plasticity, and other areas of inquiry, the intraneuronal environment also receives a great deal of investigative attention.
One example is the study of cytoskeletal polymers called neurofilaments –intermediate filaments of nerve cells that and a major component of the neuronal cytoskeleton believed to provide the axon with structural support. Neurofilaments are transported into axons where they accumulate during development, causing the axons to expand in girth. This is important because the cross-sectional area of an axon influences the rate of propagation of the nerve impulse. The space-filling properties of these polymers are maximized by spoke-like projection domains called side-arms that function to space the polymers apart. Once in the axons these polymers (which are barely 10 nm in diameter) can grow to reach remarkably long lengths – 100,000 nm (0.1 mm) or more – but how they attain such lengths and how their length is regulated is not known. Recently, scientists at The Ohio State University – who previously showed that neuro?laments and vimentin ?laments expressed in nonneuronal cell lines can lengthen by joining ends in a process known as end-to-end annealing1 – demonstrated robust and efficient end-to-end annealing of neuro?laments in nerve cells. In additions, the researchers reported evidence for a neuro?lament-severing mechanism.
Prof. Anthony Brown spoke with Medical Xpress about the research he and his colleagues – Dr. Atsuko Uchida, Dr. Gülsen Çolakoğlu (now at Sanford-Burnham Medical Research Institute), Dr. Lina Wang (now at University of California San Diego School of Medicine), and Paula C. Monsma – published in their paper. As background, Brown tells Medical Xpress that the idea that intermediate filaments can lengthen by joining at their ends was first advanced by Robert Goldman and colleagues of Northwestern University about 15 years ago, and more recently by Rudolf Leube and colleagues at Aachen University and Harald Herrmann and colleagues at the German Cancer Research Center at Heidelberg – and that computational modeling studies of vimentin assembly in vitro by Stephanie Portet at the University of Manitoba and Harald Herrrmann and colleagues also implicate end-to-end annealing in intermediate filament assembly. "However," Brown adds, "to prove that this occurs in cells it was necessary to develop experimental strategies to visualize annealing junctions – that is, to actually see the junctions. We solved this problem in a previous study1 by using cell fusion or photoactivation and photobleaching approaches to create distinct populations of red and green fluorescent filaments in cells."
For the cell fusion approach, Brown explains, using electroporation the researchers transfected cultured rat cortical neurons with fluorescent neurofilament fusion proteins so that they formed red or green fluorescent filaments. (Transfection is the introduction of a foreign material such as DNA, RNA molecules, or proteins into a eukaryotic cell.) "Then, we mixed the cells and used polyethylene glycol to induce them to fuse. For the photoactivation and photobleaching approach," he continues, "we cotransfected the cells with red and photoactivatable green fluorescent intermediate filament proteins and then used photoactivation and photobleaching to make some of the filaments red without green fluorescence and others green without red fluorescence." The scientists then observed the filaments over time.
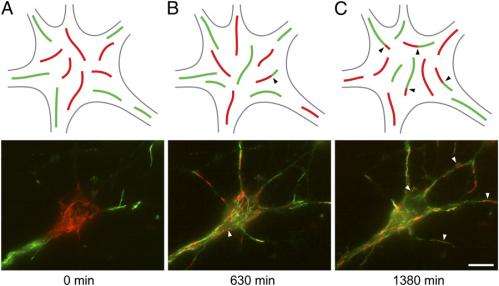
As the red and green filaments intermixed, chimeric filaments (that is, filaments composed of parts of different origin) consisting of alternating red and green fluorescent segments appeared. "The presence of red:green junctions along the filaments was proof that end-to-end annealing had occurred," notes Brown. (While the researchers performed these experiments with vimentin and neurofilament proteins, they used the SW13 human cancer cell line that does not normally express neurofilament proteins for convenience as well as technical reasons.) "However," Brown points out, "since my laboratory has a special interest in neurofilaments, we sought to confirm the existence of end-to-end annealing in nerve cells." At the same time, he adds, neurons are more challenging than cancer cell lines to work with because they're non-dividing cells – and it's also more challenging to image the filaments in neurons because they are not flattened in shape as are the cancer cells.
Finally, Brown says that the researchers could not use cell fusion strategies because the nerve cells did not tolerate the technique. Therefore, they used the photoactivation and photobleaching strategy that they had developed in their previous study1. "We also used a photoconversion strategy, taking advantage of a photoconvertible protein called mEos2, which can be switched from a green to red fluorescent form," Brown adds. "As with our earlier experiments in the human cancer cell lines, our strategy was to make distinct populations of red and green fluorescent filaments and then look for the appearance of red:green junctions."
The thickness of cultured neurons is a challenge for imaging neurofilaments, which at just 10 nm in diameter are well below the diffraction limit of the light microscope. Most of the imaging for the current study was performed with a widefield fluorescence microscope, which is not ideal because the image quality is degraded by out-of-focus fluorescence arising from above and below the plane of focus – but the scientists now have a spinning disk confocal microscope which should allow them to obtain better imaging in future studies.
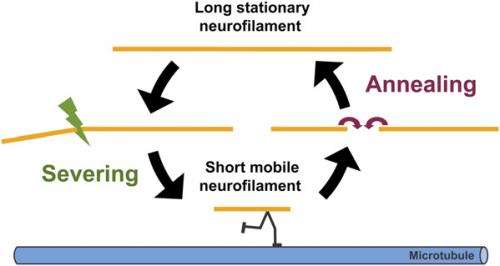
Another challenge the researchers encountered was identifying severing of neuronal intermediate filaments in vivo. "Unlike annealing, which can be visualized by the appearance of red:green junctions in our experimental strategy, there is no equivalent visual 'signature' of a severing event," Brown says. "Specifically, we can't look at the filament end and tell whether or not it arose by severing based on its appearance in the light microscope. However, we noticed that over time the increased number of red:green junctions due to end-to-end annealing was accompanied by a decrease in the lengths of the red and green fluorescent segments – the first indication that there may be a severing mechanism." The scientists were subsequently able to capture severing events live in time-lapse movies, confirming the existence of neurofilament severing.
"It also made sense to us that there should be a severing mechanism for intermediate filaments – otherwise, end-to-end annealing would result in progressively longer and longer filaments with no mechanisms to reverse that trend," Brown continues. "It seemed likely to us that the cell would have mechanisms to shorten filaments as well as lengthen them because this would give the cell more control over its intermediate filament cytoskeleton. The discovery of these two mechanisms suggested to us that neurofilament length may be regulated dynamically by their opposing actions."
Brown adds that the key innovation in the study was simply asking the right question and devising an experimental strategy to answer this question. "Little attention has been paid to intermediate filament assembly in cells, and the possible existence of a severing mechanism does not appear to have been previously addressed. In contrast, much is known about the assembly dynamics of other polymers of the cytoskeleton." In fact, he stresses, there is evidence that end-to-end annealing is an important mechanism for lengthening of actin filaments in cells, and there are well-established mechanisms for severing both actin filaments and microtubules in cells involving severing proteins such as katanin (for microtubules) and cofilin and gelsolin (for actin filaments) – but less attention has been paid to intermediate filaments. "Maybe," he quips, "no one thought to ask the question."
Brown goes into greater detail on their speculation that the elongation of neurofilaments by end-to-end annealing can be counteracted by a severing mechanism, proposing that the balance between these opposing mechanisms that regulates neurofilament length may influence neurofilaments transport within axons. "Neurofilaments are space-filling cytoskeletal polymers that function to maximize the cross-sectional area of axons, which is an important determinant of the rate of propagation of the nerve impulse," Brown explains. "Neurofilaments are also thought to play an important mechanical role in axons, conferring mechanical integrity on these long slender processes. The significance of neurofilament length for neurofilament function is not presently clear, but it seems likely that the long length of these polymers, which form a continuous overlapping array along axons, is important for their mechanical role in stabilizing internal axonal architecture."
However, he points out, it is also possible that their length may regulate their transport. "One reason for thinking this is that it is hard to conceive that neurofilaments as long as 100 µm or more could move efficiently along axons," Brown notes. "We hypothesize that neurofilament length is regulated by a dynamic cycle of severing and annealing – and we know from prior studies that there are distinct populations of moving and stationary neurofilaments, and that the average length of the moving filaments is relatively short.
Therefore, Brown continues, the scientists speculate that long and short neurofilaments coexist in axons, and that the short ones move more readily than the long ones. "Severing of long filaments may liberate short neurofilaments for axonal transport by allowing them to move along microtubule tracks. "These short mobile filaments may subsequently pause and anneal end to end, effectively sequestering them temporarily in an immobile state. One of our future goals is to test this hypothesis."
Moving forward, says Brown, the researchers need to develop methods to quantify the kinetics of annealing and severing in cells – and acknowledges this to be a challenge. "We're currently exploring the potential of computational modeling to address this problem." In addition, the researchers are very interested in the severing mechanism, Brown adds, because it could prove to be a fundamental process in intermediate filament assembly dynamics. "Intermediate filaments are known for their mechanical strength," he notes, "so how can the cell sever these polymers and how is this process regulated in cells?"
Brown also points out that there are other areas of research that might benefit from their research. "One of the central interests in my laboratory is the mechanism of axonal transport, which is the movement of intracellular components along the axons of nerve cells – and we know that neurofilament polymers move along microtubule tracks propelled by molecular motor proteins." Moreover, he adds, the axonal transport of neurofilaments is of clinical interest because neurofilament polymers often accumulate abnormally and excessively in axons in neurodegenerative disease. "We speculate that these accumulations arise by disruptions of the movement of these polymers. Our studies on the regulation of neurofilament length," he concludes, "suggest a possible mechanism by which that axonal transport of neurofilaments could be regulated."
More information: Severing and end-to-end annealing of neurofilaments in neurons, PNAS published online before print July 2, 2013, doi:10.1073/pnas.1221835110
1Intermediate filaments exchange subunits along their length and elongate by end-to-end annealing, The Journal of Cell Biology vol. 185 no. 5 769-777, May 25, 2009, doi:10.1083/jcb.200809166
© 2013 Phys.org. All rights reserved.