Study finds molecular link between gut microbes and intestinal health
It's well established that humans maintain a symbiotic relationship with the trillions of beneficial microbes that colonize their bodies. These organisms, collectively called the microbiota, help digest food, maintain the immune system, fend off pathogens, and more. There exists a long and growing list of diseases associated with changes in the composition or diversity of these bacterial populations, including cancer, diabetes, obesity, asthma, and even autism.
Inflammatory bowel disease (IBD) is one of the best-studied diseases associated with alterations in the composition of beneficial bacterial populations. However, the nature of that relationship, and how it is maintained, has yet to be clarified.
Now, researchers at the Perelman School of Medicine at the University of Pennsylvania have identified a molecule that appears to play a starring role in this process.
David Artis PhD, associate professor of Microbiology, and colleagues report in Nature that the enzyme HDAC3 is a key mediator in maintaining proper intestinal integrity and function in the presence of friendly bacteria. What's more, HDAC3 and the genetic pathways it controls appears critical to maintaining a healthy balance between intestinal microbes and their host.
"HDAC3 in intestinal epithelial cells regulates the relationship between commensal bacteria and mammalian intestine physiology," says first author Theresa Alenghat VMD PhD, instructor in the Department of Microbiology.
That humans rely on their microbial cohabitants is hardly news. Much normal human physiology is attributable to our relationship to our microbiota.
The question that Alenghat and Artis and their colleagues wanted to answer is, "What are the molecular mechanisms that control this relationship, and how is it that this relationship goes wrong and can contribute to metabolic and inflammatory diseases?"
The team focused their efforts on HDAC3, which belongs to a family of enzymes that can be responsive to environmental signals. And HDAC3 itself, an enzyme that modifies DNA and turns down gene expression, had previously been identified to have various inflammatory and metabolic roles.
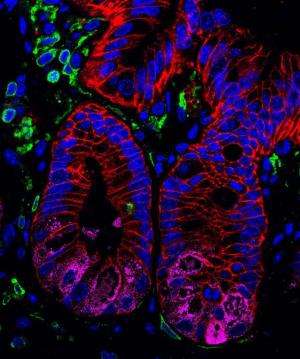
Alenghat and her colleagues looked at HDAC3 expression in normal and diseased intestine from both humans and mice, finding that the enzyme is normally expressed throughout the intestinal epithelium, but that expression is reduced in tissues from subjects with inflammatory bowel disease.
The team then developed a mouse model that would mimic that observation. They created transgenic mice that lacked HDAC3 specifically in the intestinal epithelium and found that these animals exhibited altered gene expression in their intestinal epithelial cells.
The mice also showed signs of altered intestinal health. They lacked certain cells, called Paneth cells, that produce antimicrobial peptides. The mouse intestines seemed to be more porous than normal, and they showed signs of chronic intestinal inflammation, exhibiting some of the symptoms observed in patients with IBD.
When the team examined the diversity of the microbial population colonizing the mutant animal intestines, they found they were different from normal animals, with some species being overrepresented in HDAC3-deficient mice. "There's a fundamental change in the relationship between commensal bacteria and their mammalian hosts following deletion of HDAC3 in the intestine," Artis explains.
But, if the mutant animals were grown in the absence of bacteria, their intestinal symptoms largely disappeared, as did many of the observed differences in gene expression. In other words, HDAC3 was influencing the bacterial population, and the bacteria in turn were influencing the cells' behavior.
The implication, Artis says, "is that intestinal expression of HDAC3 is an essential component of how mammals regulate the relationship between commensal bacteria and normal, healthy intestinal function."
These findings, says Alenghat, suggest a role for HDAC3 in human disease, but the exact nature of that link is still being worked out. Whether dysregulation of the enzyme, or the genetic programs it oversees, actually contributes to human IBD is a question the team is currently investigating.
"Obviously more has to be done, but it is clear that this is a pathway that is of significant interest as we continue to define how mammals have co-evolved with beneficial microbes," says Artis.
More information: Histone deacetylase 3 coordinates commensalbacteria-dependent intestinal homeostasis, DOI: 10.1038/nature12687