Vascular simulation research reveals new mechanism that switches in disease
Blood vessel formation is critical to life and its manipulation is instrumental to a number of diseases. For more than 40 years, investigations into the structure and function of endothelial cells lining the blood vessels have revealed a complex tissue with complex functions, demonstrating that endothelial cells participate in all aspects of vascular homeostasis and pathological processes.
Today, important revelations regarding endothelial cell behavior are emerging from vascular simulation research, a blossoming interdisciplinary field that makes use of novel computational models to uncover the cellular processes and dynamic interactions that take place as arteries and veins are built.
Vascular simulation research is the focus of two recent papers coauthored by investigators in the Center for Vascular Biology Research (CVBR) at Beth Israel Deaconess Medical Center (BIDMC), one of which provides a novel explanation for the enlarged blood vessels that are seen in various pathologies, including tumors and retinopathies.
"Understanding how, when and why individual endothelial cells coordinate decisions to change shape in relation to dynamic tissue environment signals is key to understanding normal and abnormal blood vessel growth," says Katie Bentley, PhD, Group Leader of the Computational Biology Laboratory in the CVBR and Assistant Professor of Pathology at Harvard Medical School. "Such insights could, for example, prove valuable in the treatment of cancer, which relies on a constant supply of blood to support tumor growth and cancerous spread."
Adaptive systems research brings things into focus
Bentley's computational modeling derives from training in Adaptive Systems research, an interdisciplinary field aimed at elucidating the fundamental organizing principles of living systems through the combined use of simulation, robotics and experimental models. Her work focuses on the construction of simulated (Simulant) endothelial cells, capable of generating novel, unexpected behavior, including movement, shape and signaling changes.
"Simulants can be observed individually and as an interacting social collective – recapitulating a growing blood vessel, for example," she explains. "We can tinker with how we set up their internal workings and then compare the changes this causes in model behavior to real cell behaviors in order to make predictions on how the mechanisms work in real cells."
Together with CVBR investigator Erzsebet Ravasz Regan, PhD, and Andrew Philippides, PhD, of the University of Sussex, UK, Bentley recently published a review article in the April 28 issue of Developmental Cell, which examines the growing field of computer simulations in vascular biology and focuses on providing an accessible account of the illuminating principles and toolsets of the Adaptive Systems field for the cell biology community.
"The Adaptive Systems field traditionally draws on animal/insect behavior and cognition to derive general principles and inspire better robotics capable of intelligent, adaptive behavior," says Bentley. "But we wanted to highlight that it can also be a very useful perspective and mindset to approach cell biology, in other words, to understand single to collective cell behavior in complex systems, such as blood vessel growth. The approach offers useful principles for studying a wide variety of questions about health and disease."
Simulant cells' unexpected behavior reveals new in vivo mechanisms
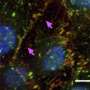
Using this type of collaborative computational and experimental approach, Bentley and a research team from the laboratory of Holger Gerhardt, PhD, of Cancer Research UK's London Research Institute recently made an unexpected discovery about endothelial cell behavior during angiogenesis. In a study published last month in Nature Cell Biology, they showed that sprouting cells are in a continuum of migratory states, regulated by differential cell-cell adhesions and protrusive activities that drive proper vascular organization.
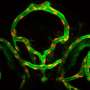
During blood vessel formation, certain molecules and cues tell endothelial cells to take on different characteristics, with some becoming "polarized tip cells" and other becoming "stalk cells." Together these two cell types form new capillaries. Tip and stalk cells were previously thought to have established roles, with tip cells leading the way and stalk cells following along to create a vessel tube, but recent research has suggested that endothelial cells are much more spontaneous and actually undergo dynamic changes and frequently switch positions, with stalk cells overtaking tip cells at the leading edge of a new blood vessel.
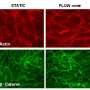
Previous studies of endothelial rearrangements suggested the adhesion molecule VE-cadherin plays a key role in helping endothelial cells to stick together. "Our team had linked rearranging behavior of endothelial cells during vessel sprouting to a signaling pathway called Notch," explains Bentley. "But what Notch regulates to generate movement and how it may interplay with VE-cadherin has remained elusive. It's also been unclear how pathological conditions such as cancer may affect the rearrangement process or whether rearrangement defects may contribute to disease."
Bentley and Gerhardt propose that when endothelial cells are stimulated by vascular endothelial growth factor (VEGF)—and not inhibited by Notch signaling – they are "active" and can either form a new branch as tip cells or "shuffle up" through the existing sprout by cell rearrangement mechanisms. Their new work in Nature Cell Biology validated simulation predictions in vivo, demonstrating that Notch regulates shuffling movement via VE-cadherin adhesion.
"We identified that feedback regulation between VEGF and Notch signaling results in different levels of adhesion [through VE-cadherin] and junctional cortex movements between cells in the sprout, which actually drives the cell rearrangement and tip cell competition," says Bentley. The team also found that during cancer development and progression, there is a switch: the regulation of the adhesion between cells becomes more uniform so that there are clustered regions of cells in an all-active or all-inhibited shuffling state.
"When we let Simulant cells loose in a simple simulated tumor environment, their collective behavior changed dramatically," says Bentley. "The cells go through cyclic phases of adhesion and junctional movements now as a group, in which they all clamber to move at once or all remain still. In either case they are getting nowhere as overtaking requires differential movement of one cell compared to its neighbors. The disrupted rearrangement provides a new explanation for the enlarged blood vessels we see in pathologies, such as in mouse models of tumors or retinopathies."
This research approach offers useful new principles for studying basic and complex questions about health and disease. "We hope that our work provides an example that cell level theory can be made accessible and, when integrated with experimentation, can help us unravel the complexities and dynamics of living systems and disease," adds Bentley.