Scientists shed new light on organ positioning in early embryos
An international team of scientists from the UK, the USA and Japan have revealed new aspects of how developing embryos establish a left and a right hand side.
While we are externally mirror symmetrical between left and right, our internal organs are asymmetrically positioned and patterned (the heart lies towards the left and the liver towards the right side of the body). This asymmetry is established between 19 and 22 days of development in humans, before the mother even knows that she is pregnant. If this process goes wrong, it can lead to birth defects and is particularly associated with congenital heart disease. New research, published in the journal PLoS Genetics provides insight into how this process happens and the ways in which it can go wrong.
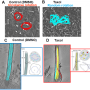
The earliest known event in mammalian left-right (L-R) patterning, surprisingly, is not an asymmetry in where a gene functions, but a physical flow of fluid (from right to left) within a short-lived pit in the embryo. This tear shaped pit is known as the node. The direction of this 'nodal flow' determines which side of the embryo will develop as the left. This leftward 'nodal flow' is driven by the action of motile cilia, hair-like structures protruding from the cell surface within the node. Exactly how nodal flow is detected by the embryo remains unclear: we know that the cells directly surrounding the node (the crown cells) are required to detect nodal flow; that the crown cells must each have an immotile cilium and that they must contain the putative calcium channel protein Polycystin-2 (PKD2). Previously the research team implicated a second Polycystin protein, PKD1L1, in this pathway.
Describing these new findings, Dominic Norris, Programme Leader at the Medical Research Council (MRC) Harwell, explains, "Firstly, we have been able to define a genetic pathway in which each element represses the next. This controls the early steps of L-R patterning: 'nodal flow' represses the gene Pkd1l1, which in turn represses Pkd2; this represses Cerl2 which encodes a known repressor of NODAL signalling (an important pathway in development of the embryo). Secondly, we demonstrate that PKD1L1 (the protein which is encoded by the Pkd1l1 gene) can mediate a response to flow. Finally, we have shown that a portion of the structure of PKD1L1 that sits outside of the cell is critical both for detecting flow and for proper L-R patterning. Together, these exciting findings reveal a genetic pathway operating at the level of flow sensation and demonstrate that PKD1L1 is able to act to elicit flow-induced chemical signals, thereby supporting the 'mechanosensation model' of nodal flow sensation (i.e. that the force of fluid flow itself can be directly detected by the node crown cells during the establishment of L-R asymmetry)".
This research has emerged from an international collaboration that has made it possible to combine genetics, biophysics and structural biology. In the UK, Rohanah Hussain and colleagues at Diamond Light Source, the UK synchrotron science facility, made it possible to understand the nature of structural changes caused by a point mutation in PKD1L1. This work took place on Diamond's Circular Dichroism beamline, B23. Surya Nauli and colleagues at Chapman University, USA, allowed the role of PKD1L1 in flow detection to be assessed in cells. Hiroshi Hamada and colleagues in Osaka, Japan, provided novel techniques that allowed the team to more precisely analyse 'nodal flow'.
Rohanah Hussain, Senior Beamline Scientist at Diamond, explains "Finding the structural changes between the normal and the mutant proteins was made challenging due to the difficulty in producing these proteins. Having limited amounts of the proteins in solution available makes the measurement difficult. Using Diamond's circular dichroism beamline, B23, this can be overcome as the synchrotron delivers micro laser-like beam and high brilliance. These unique beamline features allow small amount of samples to be used to probe the structure in solution of the two proteins showing how despite the similar folding between the two proteins, their dynamic or response behaviour were different when subjected to stress conditions mimicking the mechano-sensation property of the protein in the cell."
This work provides a greater understanding of how genes interact with a physical flow of fluid to reproducibly establish a left and right hand side in the developing embryo. This knowledge underpins our ability to understand how the process of L-R patterning can go wrong, leading to congenital diseases, including congenital heart disease.