Blocking inflammation receptor kills breast cancer stem cells, study finds

Scientists at the University of Michigan Comprehensive Cancer Center have uncovered an important link between inflammation and breast cancer stem cells that suggests a new way to target cells that are resistant to current treatments.
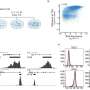
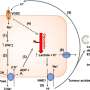
The researchers identified a receptor, CXCR1, on the cancer stem cells which triggers growth of stem cells in response to inflammation and tissue damage. A drug originally developed to prevent organ transplant rejection blocks this receptor, killing breast cancer stem cells and preventing their metastasis in mice, according to the study.
Cancer stem cells, the small number of cells that fuel a tumor's growth, are believed to be resistant to current chemotherapies and radiation treatment, which researchers say may be the reason cancer so often returns after treatment.
"Developing treatments to effectively target the cancer stem cell population is essential for improving outcomes. This work suggests a new strategy to target cancer stem cells that can be readily translated into the clinic," says senior study author Max S. Wicha, M.D., Distinguished Professor of Oncology and director of the U-M Comprehensive Cancer Center. Wicha was part of the team that first identified stem cells in breast cancer.
Results of the current study appear online Jan. 4 in the Journal of Clinical Investigation and will appear in the journal's February print issue.
CXCR1 is a receptor for Interleukin-8, or IL-8, a protein produced during chronic inflammation and tissue injury. When tumors are exposed to chemotherapy, the dying cells produce IL-8, which stimulates cancer stem cells to replicate. Addition of the drug repertaxin to chemotherapy specifically targets and kills breast cancer stem cells by blocking CXCR1.
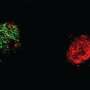
Mice treated with repertaxin or the combination of repertaxin and chemotherapy had dramatically fewer cancer stem cells than those treated with chemotherapy alone. In addition, repertaxin-treated mice developed significantly fewer metastases than mice treated with chemotherapy alone.
"These studies suggest that important links between inflammation, tissue damage and breast cancer may be mediated by cancer stem cells. Furthermore, anti-inflammatory drugs such as repertaxin may provide a means of blocking these interactions, thereby targeting breast cancer stem cells," Wicha says.
Repertaxin has been tested in early phase clinical trials to prevent rejection after organ transplantation. In these studies, side effects seem to be minimal. There are no reports of using repertaxin to treat cancer.
More information: Journal of Clinical Investigation, Vol. 120, No. 2, February 2010; doi:10.1172/JCI39397