Scientists find 'brake-override' proteins that enable development of some cancers
Scripps Research Institute scientists have discovered a basic mechanism that can enable developing cancer cells to sustain abnormal growth. The finding is expected to lead to the targeting of this mechanism with drugs and diagnostic techniques.
The study, which recently appeared in the early online edition of the Proceedings of the National Academy of Sciences, illuminates the roles of two nearly identical proteins, Cks1 and Cks2. These proteins were known to be overexpressed in many cancers, but scientists hadn't understood why. Now it appears that Cks proteins' overexpression enables cancerous growth by nullifying a natural defense against uncontrolled cell division.
"An initial cancer-promoting gene mutation can push a cell to grow faster, but the cell often detects that something is wrong and sends a signal to its DNA replication machinery to slow down," said Steven I. Reed, a professor in the Scripps Research Department of Molecular Biology and senior author of the study. "We found that when the Cks proteins are overexpressed, they cause incipient cancer cells to ignore that braking signal."
Speeding Past the Checkpoint
Reed's lab focuses on the basic biology of cell division, and Cks proteins are known to be involved in normal cell division from embryogenesis onwards. Recently, in a routine investigation of the function of Cks proteins in cells, Reed's team used a chemical known as thymidine to temporarily halt the cell division process, to artificially synchronize the growth of two different groups of cells—one with normal Cks expression, and the other with Cks overexpression. To the researchers' surprise, the Cks-overexpressing cells failed to stop dividing.
"That was a serendipitous observation," said Reed. "It led us to hypothesize that these Cks proteins, when overexpressed, are preventing cells from responding to a normal growth-braking signal."
As a dividing cell unravels its chromosomes, replicates them, and becomes two new cells, it encounters safety "checkpoints," at which the cell division process should stop if the correct signals are not in place. Thymidine inhibits cell division by producing a stop signal at what is known as the "intra-S-phase checkpoint." But somehow, Cks overexpression causes cells to speed past that checkpoint.
Reed and his team observed that the checkpoint-override effect turned out to require a Cks expression level three to four times higher than normal—the same Cks overexpression level they observed in cell lines derived from human breast tumors. "That's probably not a coincidence," said Reed.
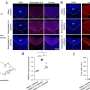
Reed's team created a simple model of Cks's function in cancer, inserting a mutant, cell-division-promoting "oncogene" into human breast-derived cells. The oncogene's protein product, known as cyclin E, pushed the cell towards uncontrolled division, which—as expected—triggered an intra-S-phase checkpoint response, so that the rate of cell division went sharply down. "When we overexpressed Cks, the cell division rate went back up again," said Reed.
Sampling real breast cancer cells from a tumor bank, Reed's team found that cells with high levels of cyclin E also tended to have high levels of Cks. Since neither protein directly influences the level of the other, the implication was that an initial oncogenic overactivation of cyclin E must in most cases be followed by Cks overexpression to keep a cell on the path to full-blown cancerous growth. "The statistical link between the cyclin-E and Cks levels was so strong that it could not have been a coincidence," said Reed.
Cks's cancer-enabling potential appears to be a broad one. When Reed's team looked at incipient cancer cells whose growth was driven by a different oncogene, h-Ras, they again found that the oncogene provoked checkpoint-dependent slower growth, whereas Cks overexpression partially nullified it and allowed cell division to proceed almost as normal.
Looking Ahead
In recent years, other researchers have noted that an initial oncogene activation can trigger a slightly different kind of growth arrest in cells. The phenomenon, known as oncogene-induced cellular senescence, is believed to underlie the slow or stopped growth of skin moles and some benign cancers. "It has been shown that this induced senescent state depends in part on the persistent activation of cell-cycle checkpoints, so presumably it is related to the process affected by Cks," said Reed.
Reed and his team are now trying to determine the precise molecular events through which Cks proteins exert their checkpoint-nullifying effect in cancer. At the same time, they are looking for ways to use their new knowledge against Cks-overexpressing cancers. The most direct strategy would be to treat cancer, or prevent it in people with inherited predispositions, simply by using a drug to reduce the activity of Cks proteins.
"We know that we can delete half of the Cks genes from mice without any deleterious effects, and this reduces the frequency of tumor formation," said Reed. "So the chances are we could find a way to reduce Cks proteins' activity enough to prevent their checkpoint-override effect while still allowing their essential cell functions."
More information: "Cyclin-dependent kinase subunit (Cks) 1 or Cks2 overexpression overrides the DNA damage response barrier triggered by activated oncoproteins," www.pnas.org/content/early/201 … /1102434108.abstract