Hair-cell-derived patient-specific heart cells for disease modeling and drug screening
Hair follicle keratinocytes offer a simple and accessible route to generate patient-specific induced pluripotent stem cells, iPSCs, with minimum inconvenience for the patients, shows study presented at the ESC Congress 2011 today.
The study presented by Dr. Katrin Streckfuss-Boemeke from Germany, won the ESC Basic Science Young Investigators Award.
"Data gathered in this study demonstrates an easy and fast possibility to generate iPSCs from hair follicles of patients with genetic cardiac diseases and their further differentiation into functional cardiomyocytes. These cells will allow us to model the heart disease of these patients, to investigate the mechanisms of the disease, to perform drug screenings and to develop patient-specific therapeutic strategies," explained Dr Streckfuss-Boemeke.
Most iPSC described in previous studies were generated from skin fibroblasts or bone marrow cells which require surgical intervention. The aim of this study was to use an alternative cell source that could be readily and noninvasively isolated from patients and which shows a high proliferation rate for efficient reprogramming.
Heart disease is one of the leading causes of death in developed countries. Although the majority of cardiac death victims are elderly, many children and young adults under the age of 35 die each year due to various cardiac pathologies. Recent progress in the fields of molecular biology and human genetics have enabled identification of the genetic causes of many heart diseases, including multiple causes of sudden cardiac death (SCD).
Genetically determined cardiac diseases can be divided into two groups : diseases with a structural change of the heart, like hypertrophic cardiomyopathy (HCM) or arrhythmogenic right ventricular dysplasia (ARVD) and a second group, without structural changes, resulting in life-threatening cardiac arrhythmia. Examples are primary electric heart diseases (the channelopathies), as the long QT syndrome (LQTS), the short QT syndrome (SQTS), the Brugada syndrome (BrS) and the Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT), which causes sudden unexpected cardiac death in apparently healthy young individuals.
Several genetic cardiac diseases are not rare, for example HCM (although it is most common cause of SCD in childhood), LQTS and BrS. However, several diseases are rare, for example, CPVT and ARVD, and are very often related to high mortality in children and young adults. The cumulative mortality of the CPVT cases is 30-50% by the age of 20-30 years.
Until now, most of studies of human cardiac developmental defects or adult diseases have been done in animal models, especially in mice. However, mice differ from humans in many ways; for example, a mouse heart beats at 500 beats per minute, while the human heart beats at 70 beats per min. Therefore, the use of pluripotent stem cells is a promising approach for studying cardiac diseases.
Pluripotent stem cells have two major properties: they can proliferate indefinitely and they can form about 220 different cell types present in the adult body, including functional cardiomyocytes. Lately, the reprogramming of somatic cells to induced pluripotent stem cells (so called iPSCs) has become very popular. For the pluripotency induction, proteins (e.g. the pluripotency factors Oct4, Sox2, Nanog, Lin28 Klf4, and cMyc) are introduced into the cells. Therefore, human iPSC cells offer the particularly attractive opportunity to convert somatic cells from patients with cardiac disease into iPSCs and differentiate these iPSCs into patient-specific cardiomyocytes. In this way we can establish a human heart disease model for analyzing molecular mechanisms and developing individual therapy strategies. This would not be possible by using heart biopsies from living patients, because of limitation regarding the procedure and the amount of the taken tissues.
However, most iPSC lines described in previous studies have been generated from skin fibroblasts or bone marrow cells that require surgical intervention on the patient for their isolation. Therefore, our aim was to use an alternative cell source that could be readily and noninvasively isolated from patients and shows a high proliferation rate for efficient reprogramming. We decided to use keratinocytes, which can be easily isolated from the hair of living human individuals.
Previous studies showed that hair-plucked keratinocytes are difficult to be cultivated with a low proliferation capacity. Therefore our first aim was the optimization of culture conditions for keratinocytes from human hair follicles. We plucked approximately 40 hairs from 41 individuals between 22-52 years, isolated and cultivated hair follicle keratinocytes with an efficiency of 90%. After 10 to 14 days we had a sufficient amount of keratinocytes in culture and started the induction of reprogramming of these hair cells into pluripotent stem cells. We introduced different combinations of pluripotency genes (Oct4, Sox2, Nanog, and Lin28 (OSNL) or Oct, Sox2, Klf4, and cMyc (OSKM) by using the lentiviral system. Oct4 and Sox2 are two transcription factors, which act as transcriptional activator of other pluripotency-associated genes. Nanog is more involved in the maintenance of pluripotency, and Lin28 for example has been used to enhance the efficiency of reprogramming. After 2-3 weeks we saw iPSC-colonies (Kera-iPSCs) in culture with a typical compact morphology of pluripotent human embryonic stem cells (hESCs).
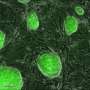
We performed three sets of experiments for proving the pluripotency of the generated kera-iPSCs: First we analyzed the expression of typical pluripotency markers on gene and protein levels. We observed that the endogenous expression of OCT4 and SOX2 is very low in keratinocytes in contrast to a significantly increase in the keratinocyte-derived iPSCs. We detected no endogenous expression of NANOG and LIN28 in the keratinocytes but a highly induction in the iPSCs. These gene expression data were confirmed on the protein level. We were able to show that the kera-iPSCs were positive for markers common to pluripotent cells, including alkaline phosphatase, Nanog, Oct4, Sox2, Tra-1-60, and SSEA4.
The second proof of pluripotency of the generated iPSCs is their differentiation potential in vivo and therefore the ability to form teratomas. To test this, we injected the cells subcutaneously in immune-suppressed SCID-beige mice. After 8 weeks we observed the formation of teratomas, in which differentiating cells of all three germ layers were found, including cartilage, epithelium with intestinal differentiation and neural tissues.
In a last set of experiments we analysed the iPSC differentiation potential in vitro. Using the so-called embryoid body (EB) formation, we induced the cells to differentiate spontaneously into derivatives of all three embryonic germ layers in vitro.
During this time in vitro, tissue-specific genes were expressed in a developmentally controlled manner, including AFP-positive hepatic cells, tyrosine-hydroxylase positive neuronal cells and troponinT-positive cardiac cells. But the most exciting observation during this differentiation is the rhythmically beating clusters in EB outgrowths.
All these experiments show that the hair keratinocyte-derived iPSCs are really pluripotent and can be differentiated into beating cardiomyocytes.
These data demonstrate an easy and fast possibility to generate iPSCs from hair follicles of patients with genetic cardiac diseases and their further differentiation into functional cardiomyocytes. In comparison to hair-follicle-derived cardiomyocytes from healthy individuals we will be able to model the heart disease, to investigate the mechanisms of the disease, to perform drug screenings and to develop patient-specific therapeutic strategies (Fig.1). In conclusion, hair follicle keratinocytes offer a simple and accessible route to generate patient-specific iPSCs, and with minimum inconvenience for the patients.