New findings about the prion protein and its interaction with the immune system
(Medical Xpress) -- Scrapie is a neurodegenerative disease which can function as a model for other diseases caused by an accumulation of proteins resulting in tissue malformations (proteinpathies), such as Alzheimer's and Parkinson's disease.
Many questions regarding these diseases still remain unanswered. A new doctoral study has uncovered a number of factors relating to the uptake of the prion protein (PrPSc) associated with the development of this disease and how this protein interacts with the immune cells in the intestines.
Scrapie in sheep belongs to a group of diseases called ”Transmissible spongiform encephalopathies”(TSE) because they are transmitted between individual animals and produce sponge-like, degenerative changes in the brain. These diseases afflict not only sheep but also cattle (BSE), deer (CWD) and humans (CJD). They can to a certain extent also be transmitted between species, as was the case during the 1990s, when over 200 people were infected by food and contracted CJD.
TSE, otherwise known as prion diseases, are thought to be transmitted by means of a diseased variant of a protein, the prion protein, which is a normal component of body cells and is most prolific in the brain. Generally speaking, prion diseases may be infectious, hereditary or occur sporadically/spontaneously. Disease arises when the normal prion protein mutates to the diseased variant, which differs from the healthy prion proteins by its change in structure. The body's cells have difficulty in breaking down this prion protein due to its different structure, and it therefore accumulates.
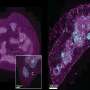
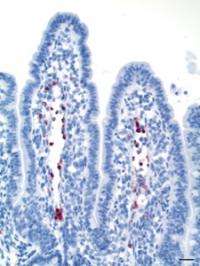
Since PrPSc is to be found in the lymphatic tissue of the intestinal system at an early stage of the disease, it is assumed that transmission occurs via the gastrointestinal tract. During her doctoral research, veterinary scientist Caroline Piercey Åkesson studied the uptake of the prion protein in the intestines, thereby throwing new light on processes occurring during the early phase of the disease's development. Contrary to earlier assumptions, she demonstrated by means of immunoelectron microscopy that the prion protein responsible for the disease is not transported directly from the intestines to lymphatic tissue associated with the intestines. On the contrary, she showed that the protein passed freely or in lymphatic cells outside the organised lymphatic tissue in the intestines.
Dendritic cells are presumed to function as "gatekeepers" which determine what the body can tolerate and which immune defence reactions it needs to instigate when confronted with foreign substances. One of the objectives of Åkesson's project was therefore to examine the interaction of dendritic cells with prion protein uptake. Firstly, it was necessary to characterise dendritic cells in healthy sheep intestines and secondly, to investigate which types of cells were associated with the uptake of the prion protein.
Her findings showed that it was not dendritic cells, but macrophages, which were mainly responsible for the uptake of the protein. Åkesson's study revealed that the prion protein makes use of the normal physiological uptake channel for macromolecules in the intestines and that this may have a significant effect on the body's immunological surveillance system. One possible consequence is that immuno-tolerance is stimulated, thus impeding a normal immuno reaction against the prion protein absorbed via the intestines.
Future studies which can reveal how immunological cells are transported and how the prion protein is processed in the body will be of great interest, not only in order to provide more knowledge about scrapie, but also about other neurodegenerative proteinpathies, both in humans and animals.
Cand.med.vet. Caroline Piercey Åkesson defended her doctoral thesis on 20th December 2011 at The Norwegian School of Veterinary Science. The thesis is entitled: Studies on the uptake of prions and their early interaction with immune cells of the sheep gut.