A new approach to treating type I diabetes? Gut cells transformed into insulin factories
A study by Columbia researchers suggests that cells in the patient's intestine could be coaxed into making insulin, circumventing the need for a stem cell transplant. Until now, stem cell transplants have been seen by many researchers as the ideal way to replace cells lost in type I diabetes and to free patients from insulin injections.
The research—conducted in mice—was published 11 March 2012 in the journal Nature Genetics.
Type I diabetes is an autoimmune disease that destroys insulin-producing cells in the pancreas. The pancreas cannot replace these cells, so once they are lost, people with type I diabetes must inject themselves with insulin to control their blood glucose. Blood glucose that is too high or too low can be life threatening, and patients must monitor their glucose several times a day.
A longstanding goal of type I diabetes research is to replace lost cells with new cells that release insulin into the bloodstream as needed. Though researchers can make insulin-producing cells in the laboratory from embryonic stem cells, such cells are not yet appropriate for transplant because they do not release insulin appropriately in response to glucose levels. If these cells were introduced into a patient, insulin would be secreted when not needed, potentially causing fatal hypoglycemia.
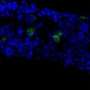
The study, conducted by Chutima Talchai, PhD, and Domenico Accili, MD, professor of medicine at Columbia University Medical Center, shows that certain progenitor cells in the intestine of mice have the surprising ability to make insulin-producing cells. Dr. Talchai is a postdoctoral fellow in Dr. Accili's lab.
The gastrointestinal progenitor cells are normally responsible for producing a wide range of cells, including cells that produce serotonin, gastric inhibitory peptide, and other hormones secreted into the GI tract and bloodstream.
Drs. Talchai and Accili found that when they turned off a gene known to play a role in cell fate decisions—Foxo1—the progenitor cells also generated insulin-producing cells. More cells were generated when Foxo1 was turned off early in development, but insulin-producing cells were also generated when the gene was turned off after the mice had reached adulthood.
"Our results show that it could be possible to regrow insulin-producing cells in the GI tracts of our pediatric and adult patients," Dr. Accili says.
"Nobody would have predicted this result," Dr. Accili adds. "Many things could have happened after we knocked out Foxo1. In the pancreas, when we knock out Foxo1, nothing happens. So why does something happen in the gut? Why don't we get a cell that produces some other hormone? We don't yet know."
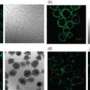
Insulin-producing cells in the gut would be hazardous if they did not release insulin in response to blood glucose levels. But the researchers say that the new intestinal cells have glucose-sensing receptors and do exactly that.
The insulin made by the gut cells also was released into the bloodstream, worked as well as normal insulin, and was made in sufficient quantity to nearly normalize blood glucose levels in otherwise diabetic mice.
"All these findings make us think that coaxing a patient's gut to make insulin-producing cells would be a better way to treat diabetes than therapies based on embryonic or iPS stem cells," Dr. Accili says. The location of the cells in the gut may also prevent the diabetes from destroying the new insulin-producing cells, since the gastrointestinal tract is partly protected from attack by the immune system.
The key to turning the finding into a viable therapy, Dr. Accili says, will be to find a drug that has the same effect on the gastrointestinal progenitor cells in people as knocking out the Foxo1 gene does in mice. That should be possible, he says, since the researchers found that they could also create insulin-producing cells from progenitor cells by inhibiting Foxo1 with a chemical.
"It's important to realize that a new treatment for type I diabetes needs to be just as safe as, and more effective than, insulin," Dr. Accili says. "We can't test treatments that are risky just to remove the burden of daily injections. Insulin is not simple or perfect, but it works and it is safe."