Scientists turn patients' skin cells into heart muscle cells to repair their damaged hearts
For the first time scientists have succeeded in taking skin cells from heart failure patients and reprogramming them to transform into healthy, new heart muscle cells that are capable of integrating with existing heart tissue.
The research, which is published online today (Wednesday) in the European Heart Journal, opens up the prospect of treating heart failure patients with their own, human-induced pluripotent stem cells (hiPSCs) to repair their damaged hearts. As the reprogrammed cells would be derived from the patients themselves, this could avoid the problem of the patients' immune systems rejecting the cells as "foreign". However, the researchers warn that there are a number of obstacles to overcome before it would be possible to use hiPSCs in humans in this way, and it could take at least five to ten years before clinical trials could start.
Recent advances in stem cell biology and tissue engineering have enabled researchers to consider ways of restoring and repairing damaged heart muscle with new cells, but a major problem has been the lack of good sources of human heart muscle cells and the problem of rejection by the immune system. Recent studies have shown that it is possible to derive hiPSCs from young and healthy people and that these are capable of transforming into heart cells. However, it has not been shown that hiPSCs could be obtained from elderly and diseased patients. In addition, until now researchers have not been able to show that heart cells created from hiPSCs could integrate with existing heart tissue.
Professor Lior Gepstein, Professor of Medicine (Cardiology) and Physiology at the Sohnis Research Laboratory for Cardiac Electrophysiology and Regenerative Medicine, Technion-Israel Institute of Technology and Rambam Medical Center in Haifa, Israel, who led the research, said: "What is new and exciting about our research is that we have shown that it's possible to take skin cells from an elderly patient with advanced heart failure and end up with his own beating cells in a laboratory dish that are healthy and young – the equivalent to the stage of his heart cells when he was just born."
Ms Limor Zwi-Dantsis, who is a PhD student in the Sohnis Research Laboratory, Prof Gepstein and their colleagues took skin cells from two male heart failure patients (aged 51 and 61) and reprogrammed them by delivering three genes or "transcription factors" (Sox2, Klf4 and Oct4), followed by a small molecule called valproic acid, to the cell nucleus. Crucially, this reprogramming cocktail did not include a transcription factor called c-Myc, which has been used for creating stem cells but which is a known cancer-causing gene.
"One of the obstacles to using hiPSCs clinically in humans is the potential for the cells to develop out of control and become tumours," explained Prof Gepstein. "This potential risk may stem from several reasons, including the oncogenic factor c-Myc, and the random integration into the cell's DNA of the virus that is used to carry the transcription factors – a process known as insertional oncogenesis."
The researchers also used an alternative strategy that involved a virus that delivered reprogramming information to the cell nucleus but which was capable of being removed afterwards so as to avoid insertional oncogenesis.
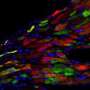
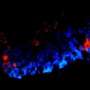
The resulting hiPSCs were able to differentiate to become heart muscle cells (cardiomyocytes) just as effectively as hiPSCs that had been developed from healthy, young volunteers who acted as controls for this study. Then the researchers were able to make the cardiomyocytes develop into heart muscle tissue, which they cultured together with pre-existing cardiac tissue. Within 24-48 hours the tissues were beating together. "The tissue was behaving like a tiny microscopic cardiac tissue comprised of approximately 1000 cells in each beating area," said Prof Gepstein.
Finally, the new tissue was transplanted into healthy rat hearts and the researchers found that the grafted tissue started to establish connections with the cells in the host tissue.
"In this study we have shown for the first time that it's possible to establish hiPSCs from heart failure patients – who represent the target patient population for future cell therapy strategies using these cells – and coax them to differentiate into heart muscle cells that can integrate with host cardiac tissue," said Prof Gepstein.
"We hope that hiPSCs derived cardiomyocytes will not be rejected following transplantation into the same patients from which they were derived. Whether this will be the case or not is the focus of active investigation. One of the obstacles in dealing with this issue is that, at this stage, we can only transplant human cells into animal models and so we have to treat the animals with immunosuppressive drugs so the cells won't be rejected."
Much research has to be conducted before these results could be translated into treatment for heart failure patients in the clinic. "There are several obstacles to clinical translation," said Prof Gepstein. "These include: scaling up to derive a clinically relevant number of cells; developing transplantation strategies that will increase cell graft survival, maturation, integration and regenerative potential; developing safe procedures to eliminate the risks for causing cancer or problems with the heart's normal rhythm; further tests in animals; and large industry funding since it is likely to be a very expensive endeavour. I assume it will take at least five to ten years to clinical trials if one can overcome these problems."
Prof Gepstein and his colleagues will be carrying out further research into some of these areas, including evaluating using hiPSCs in cell therapy and tissue engineering strategies for repairing damaged hearts in various animal models, investigating inherited cardiac disorders, and drug development and testing.
Editor-in-Chief of the European Heart Journal, Professor Thomas Lüscher, who is Professor and Chairman of Cardiology at the University Hospital Zurich and Director of Cardiovascular Research at the Institute of Physiology of the University Zurich, Switzerland, commented: "The European Heart journal is proud to publish this exciting study which opens the door for a novel approach in regenerative medicine."
More information: "Derivation and cardiomyocyte differentiation of induced pluripotent stem cells from heart failure patients," by Limor Zwi-Dantsis, Irit Huber, Manhal Habib, Aaron Winterstern, Amira Gepstein, Gil Arbel, and Lior Gepstein. European Heart Journal. doi:10.1093/eurheartj/ehs096