Primitive cell populations retained from early embryonic development could provide seeds for precancerous growths
Heartburn makes for an uncomfortable post-meal experience, but can also herald more serious health concerns. Indeed, gastroesophageal reflux disease (GERD) is a causative factor underlying Barrett’s metaplasia, a condition associated with changes in the epithelial cells lining the esophagus that can ultimately lead to esophageal carcinoma.
Esophageal carcinoma incidence has increased over five-fold in the Western world since 1970, but little is known about its etiology. “The fact that the five-year survival rate has not appreciably changed during this time is demoralizing and suggests that surgery, radiation and chemotherapy have not made a dent in our ability to manage this disease,” says Frank McKeon at the A*STAR Genome Institute of Singapore. However, patients may draw hope from new findings obtained by a team led by McKeon, and Wa Xian of the A*STAR Institute of Medical Biology, using a genetically-modified mouse strain that models Barrett’s metaplasia.
These animals, lacking a protein called p63, exhibited metaplasia in the epithelial cells where the esophagus meets the stomach. The team, led by McKeon and Xian, observed striking changes in gene expression in this subset of cells, with a strong correlation in the genes that were differentially expressed among p63-deficient mouse tissues and samples from human patients with Barrett’s metaplasia.
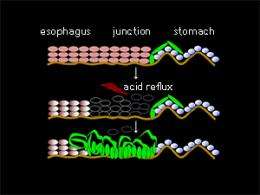
The researchers identified two marker proteins that allowed them to visualize the cells that contribute to metaplasia during embryonic development. In wild-type mice, these cells initially line the stomach, but subsequently get displaced by the expansion of a population of p63-expressing epithelial cells. In p63-deficient animals, however, these embryonic epithelial cells linger and turn metaplastic.
Importantly, wild-type mice still retain a population of these cells, dubbed ‘residual embryonic cells’ (RECs), in the stomach region adjacent to the esophagus, and analysis of human tissues revealed a similar population of RECs at the gastroesophageal junction. The researchers hypothesize that these cells represent an ‘opportunistic’ population that is normally prevented from proliferating by fully mature ‘indigenous’ epithelia. By inducing damage to this mature epithelium, GERD may thus allow RECs to proliferate unchecked (see image).
The findings suggest that Barrett’s esophagus does not arise from the typical activating mutations seen in early precursors of cancers, but rather exploits damage to the esophagus in order to expand and grow. This ‘rogue cell’ model could potentially underlie other cancers, and the researchers are now examining whether it is possible to avert esophageal carcinoma by selectively eliminating RECs. “We are identifying unique cell surface targets for targeted therapy to destroy these cells,” say Xian.
More information: Wang, X. et al. Residual embryonic cells as precursors of a Barrett’s-like metaplasia. Cell 145, 1023–1035 (2012). dx.doi.org/10.1016/j.cell.2011.05.026