Predicting toxicity in the drug development pipeline

University College Dublin researchers have reported in Molecular & Cellular Proteomics on a proof-of-principle study that may benefit the pharmaceutical industry in the future by providing a roadmap for large scale pre-clinical toxicology biomarker verification studies.
It is not currently possible to predict accurately and at an early stage whether there are toxicity issues with candidate drugs. This shortcoming of existing toxicology evaluation methods can not only create a bottleneck in the drug development pipeline but can sometimes lead to the withdrawal of drugs from the market.
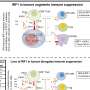
The study involved the molecular profiling of models that had been exposed to known toxic insults in an effort to derive the associated biomarker signatures. Forty-eight candidate biomarkers of liver toxicity were assembled from a discovery proteomics screen of liver in a hepatotoxicant treated rat model using label free liquid chromatography mass spectrometry (LC-MS); a previous transcriptomics study of the sample samples and from literature sources.
The team developed and optimised a selected reaction monitoring assay (SRM) in order to quantify the proteins in this putative biomarker panel. This revealed a panel highly enriched for proteins that had been changed significantly as a result of toxicant exposure .
Dr. Ben Collins, first author and Agilent UCD Newman Fellow, explains, “The idea was to use transcriptomics, and proteomics and to combine the data to provide earlier markers toxicity. Although this study focused on one hepatotoxic compound, there is sufficient flexibility in the approach used to allow medium to high throughput for large scale verification studies involving large numbers of well- defined toxicants and ultimately for more sensitive toxicology evaluation for drugs under early development”.
Team leader and corresponding author, Professor Steve Pennington adds, “We are now working to extend this approach to more readily accessible sample types, such as blood, and are applyijng it in other studies for diagnostics for chronic conditions, such as cancer, cardiovascular disease, and arthritis. With supporti from Agilent are now establishing a dedicated lab to undertake these SRM-based validation studies”.
More information: Collins, BC et al; Development of a pharmaceutical hepatotoxicity biomarker panel using a discovery to targeted proteomics approach. Molecular & Cellular Proteomics (2012) doi: 10.1074/mcp.M111.016493