Cancer gene family member functions key to cell adhesion and migration
The WTX gene is mutated in approximately 30 percent of Wilms tumors, a pediatric kidney cancer. Like many genes, WTX is part of a family. In this case, WTX has two related siblings, FAM123A and FAM123C. While cancer researchers are learning more of WTX and how its loss contributes to cancer formation, virtually nothing is known of FAM123C or FAM123A, the latter of which is a highly abundant protein within neurons, cells that receive and send messages from the body to the brain and back to the body.
A UNC-led team of scientists used sophisticated technologies to identify and describe the protein interactions that distinguish each member of the WTX family. They found that unlike WTX and FAM123C, FAM123A interacts with a specific set of proteins that regulates cell adhesion and migration, processes essential to normal cell functioning and which, when mutated, contribute to human diseases such as cancer or Alzheimer's.
The report is the first to associate a member of the WTX gene family with cell adhesion and migration. Ben Major, PhD, and his research team believe that because FAM123A is so highly expressed in neurons, their findings raise the possibility that FAM123A controls neuron migration and neuronal activity, both of which play critical roles in development, neuro-degeneration and learning. Dr. Major, study senior author, is an assistant professor of cell biology and physiology in the UNC School of Medicine and a member of UNC Lineberger Comprehensive Cancer Center.
Their report appears in the September 4, 2012 online edition of Science Signaling.
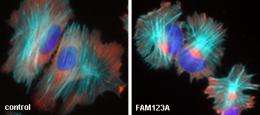
The specific set of proteins the scientists discovered within the FAM123A complex are known microtubules-associated proteins. Microtubules, one component of a cell's cytoskeleton, are rigid hollow rods approximately 25 nm in diameter, about 3000 times thinner than a human hair. Microtubules are dynamic structures that undergo continual assembly and disassembly within the cell. They function both to determine cell shape and to control a variety of cell movements, including some forms of cell locomotion.
Dr. Major explains, "Since FAM123A and WTX are closely related proteins, anything we learn about FAM123A helps us know more about WTX, the tumor suppressor gene lost in pediatric kidney cancer. Unlike WTX, FAM123A binds to a specific set of proteins that are famous for regulating microtubules, a critical component within the cell's cytoskeleton or cellular 'scaffolding.' It's important to understand how different cellular cytoskeletal networks communicate and coordinate with each other. This communication is required for normal development and life.
"When the cytoskeleton is not functioning properly, a myriad of diseases arise, including certain cancers and cancer metastases. In cancer, cells can't move to new areas of the body without being able to squeeze between and crawl around surrounding cells and tissues, which ultimately allows the cell to move away from the primary tumor. Such movement requires complicated and intricate coordination between the cytoskeleton and the rest of the cell. Our work shows that FAM123A is critical for this communication."
Dr. Major and his research team use a powerful new technology to study protein complexes. He says, "Proteins never work alone, rather they bind each other to collectively carry out a specific task. An important challenge in cancer research today is determining which of the more than 30,000 proteins in a cell come together to form complexes." Dr. Major and colleagues can purify a specific protein from cancer cells and then, using sophisticated technologies called mass spectrometry, they identify the associated proteins.













