Chemists determine one way tumors meet their growing need
Behaving something like ravenous monsters, tumors need plentiful supplies of cellular building blocks such as amino acids and nucleotides in order to keep growing at a rapid pace and survive under harsh conditions. How such tumors meet these burgeoning demands has not been fully understood. Now chemists at the California Institute of Technology (Caltech) have shown for the first time that a specific sugar, known as GlcNAc ("glick-nack"), plays a key role in keeping the cancerous monsters "fed." The finding suggests new potential targets for therapeutic intervention.
The new results appear in this week's issue of the journal Science.
The research team—led by Linda Hsieh-Wilson, professor of chemistry at Caltech—found that tumor cells alter glycosylation, the addition of carbohydrates (in this case GlcNAc) to their proteins, in response to their surroundings. This ultimately helps the cancerous cells survive. When the scientists blocked the addition of GlcNAc to a particular protein in mice, tumor-cell growth was impaired.
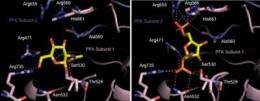
The researchers used chemical tools and molecular modeling techniques developed in their laboratory to determine that GlcNAc inhibits a step in glycolysis (not to be confused with glycosylation), a metabolic pathway that involves 10 enzyme-driven steps. In normal cells, glycolysis is a central process that produces high-energy compounds that the cell needs to do work. But Hsieh-Wilson's team found that when GlcNAc attaches to the enzyme phosphofructokinase 1 (PFK1), it suppresses glycolysis at an early phase and reroutes the products of previous steps into a different pathway—one that yields the nucleotides a tumor needs to grow, as well as molecules that protect tumor cells. So GlcNAc causes tumor cells to make a trade—they produce fewer high-energy compounds in order to get the products they need to grow and survive.
"We have identified a novel molecular mechanism that cancer cells have co-opted in order to produce intermediates that allow them to grow more rapidly and to help them combat oxidative stress," says Hsieh-Wilson, who is also an investigator with the Howard Hughes Medical Institute.
This is not the first time scientists have identified a mechanism by which tumor cells might produce the intermediates they need to survive. But most other mechanisms have involved genetic alterations, or mutations—permanent changes that lead to less active forms of enzymes, for example. "What's unique here is that the addition of GlcNAc is dynamic and reversible," says Hsieh-Wilson. "This allows a cancer cell to more rapidly alter its metabolism depending on the environment that it encounters."
In their studies, Hsieh-Wilson's team found that this glycosylation—the addition of GlcNAc to PFK1—is enhanced under conditions associated with tumors, such as low oxygen levels. They also found that glycosylation of PFK1 was sensitive to the availability of nutrients. If certain nutrients were absent, glycosylation was increased, and the tumor was able to compensate for the dearth of nutrients by changing the cell's metabolism.
When the researchers analyzed human breast and lung tumor tissues, they found GlcNAc-related glycosylation was elevated two- to fourfold in the majority of tumors relative to normal tissue from the same patients. Then, working with mice injected with human lung-cancer cells, the researchers replaced the existing PFK1 enzymes with either the normal PFK1 enzyme or a mutant form that could no longer be glycosylated. The mice with the mutant form of PFK1 showed decreased tumor growth, demonstrating that blocking glycosylation impairs cancerous growth.
The work suggests at least two possible avenues for future investigations into fighting cancer. One would be to develop compounds that prevent PFK1 from becoming glycosylated, similar to the mutant PFK1 enzymes in the present study. The other would be to activate PFK1 enzymes in order to keep glycolysis operating normally and help prevent cancer cells from altering their cellular metabolism in favor of cancerous growth.
Hsieh-Wilson's group has previously studied GlcNAc-related glycosylation in the brain. They have demonstrated, for example, that the addition of GlcNAc to a protein called CREB inhibits the protein's ability to turn on genes needed for long-term memory storage. On the other hand, they have also shown that having significantly lower levels of GlcNAc in the forebrain leads to neurodegeneration. "The current thinking is that there's a balance between too little and too much glycosylation," says Hsieh-Wilson. "Being at either extreme make things go awry, whether it's in the brain or in the case of cancer cells."