Pre-op eltrombopag reduces need for platelet transfusions
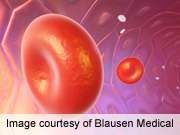
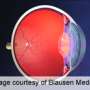
(HealthDay)—For patients with chronic liver disease who require an invasive procedure as part of their routine care, the oral thrombopoietin-receptor agonist eltrombopag reduces the need for platelet transfusions, but also results in an increased incidence of portal-vein thrombosis, according to a study published in the Aug. 23 issue of the New England Journal of Medicine.
Nezam H. Afdhal, M.D., from the Beth Israel Deaconess Medical Center in Boston, and colleagues randomly assigned 292 patients with chronic liver disease and platelet counts of less than 50,000 per cubic millimeter to receive either eltrombopag (75 mg daily) or placebo for 14 days before a planned elective invasive procedure, performed within five days of receiving the last dose.
The researchers found that platelet transfusion (before, during, and up to seven days after surgery) was avoided in 104 of 145 patients who received eltrombopag and in 28 of 147 who received placebo (72 versus 19 percent; P < 0.001). There were no significant differences in bleeding episodes of World Health Organization grade 2 or higher, which were reported in 17 percent of the eltrombopag group and 23 percent of the placebo group. Six patients receiving eltrombopag had thrombotic events of the portal venous system, compared with one patient who received placebo, which triggered early termination of the study. Other adverse events were similar in incidence and severity between the groups.
"Eltrombopag reduced the need for platelet transfusions in patients with chronic liver disease who were undergoing elective invasive procedures, but it was associated with an increased incidence of portal-vein thrombosis, as compared with placebo," the authors write.
Several authors disclosed financial ties to pharmaceutical companies, including GlaxoSmithKline, which funded the study and manufactures eltrombopag.
More information: Full Text (subscription or payment may be required)
Copyright © 2012 HealthDay. All rights reserved.