Epigenetic causes of prostate cancer
(Medical Xpress)—In about half of all prostate tumours, there are two genetic areas that are fused with one another. When this is not the case, the exact way cancer cells originate in prostate tumours was not clear until now. Scientists at the Max Planck Institute for Molecular Genetics in Berlin, in cooperation with a team of international researchers, were able to show that the genesis of this fusion-negative prostate cancer has epigenetic causes: methyl groups are distributed differently over the DNA in the cancer cells than in healthy cells. Thanks to this knowledge, physicians may be able to achieve greater specificity in treating prostate tumours in future. In addition, the aberrant DNA methylations can be used as a potential biomarker for identifying prostate cancer.
About half of all cases of prostate cancer originate through fusion of two genetic areas. As a result, the ERG gene is activated in these fusion-positive cells and prostate cells propagate, leading to tumourigenesis. Fusion-positive prostate cancer can be treated with PARP1 inhibitors that turn off the repair system of the tumour cells.
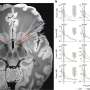
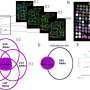
However, it has not been clear how prostate tumours without a fused ERG gene acquire their tumourigenic potential. Now, a team of Max Planck scientists headed by Michal-Ruth Schweiger from the Department of Vertebrate Genomics have investigated the global DNA methylation pattern – i.e. at which locations the DNA possesses methyl groups – in fusion-negative tumours. They have discovered that, compared to fusion-positive tumours, the fusion-negative tumours display more aberrant DNA methylations, which are most likely causative for the malignant transformation of prostate cells.
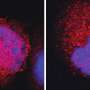
Moreover, the scientists found large amounts of the enzyme EZH2 in the cells of the tumours. This histone methyltransferase couples histone and DNA methylation and also transfers methyl groups to the DNA. Further functional analyses link EZH2 to the aberrant DNA methylations in fusion gene negative tumours. As reason for the significantly increased amount of EZH2 the scientists found very low concentrations of miRNA-26a, a micro-RNA that targets EZH2 for degradation.
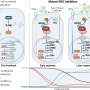
The researchers think that their findings will further enhance diagnosis and specific treatment options for prostate cancer patients. "Regions with different DNA-methylation patterns can be used as biomarkers to diagnose specific subgroups of cancer", says Schweiger. "In addition, new medications for fusion-negative prostate cancer could operate with greater specificity and therefore be more effective." It is likely that other types of cancer are also based on these kinds of pronounced epigenetic modifications.
More information: Börno ST, et al. Genome-wide DNA methylation events in TMPRSS2:ERG fusion negative prostate cancers implicate an EZH2 dependent mechanism with miRNA-26a hypermethylation. Cancer Discovery, Published Online First August 28, 2012; doi: 10.1158/2159-8290.CD-12-0041