Alzheimer drug shows some promise in mild disease (Update)
Combined results from two studies of an experimental Alzheimer's drug suggest it might modestly slow mental decline, especially in patients with mild disease.
Taken separately, the studies on the drug—Eli Lilly & Co.'s solanezumab—missed their main goals of significantly slowing the mind-robbing disease or improving activities of daily living. But pooled results found 34 percent less mental decline in mild Alzheimer's patients compared to those on a fake treatment for 18 months.
Doctors called the results encouraging although probably not good enough to win approval of the drug now, without another study to confirm there is a benefit. Investors were more enthused, driving Lilly's stock up about 5 percent on Monday and about 19 percent since August, when the company described the results in general terms.
Detailed results were revealed for the first time Monday at an American Neurological Association conference in Boston.
"It's certainly not the home run we all wanted, but we're very encouraged by these results," said Maria Carillo, chief science officer for the Alzheimer's Association, which had no role in the research.
Dr. Stephen Salloway, an Alzheimer's expert at Brown University, agreed.
"It's exciting to see that there may be clinical benefit," he said, but it is modest and may not make a difference in how well patients live—what matters most to them and their families, he said.
About 35 million people worldwide have dementia, and Alzheimer's is the most common type. In the U.S., about 5 million have Alzheimer's. Current medicines such as Aricept and Namenda just temporarily ease symptoms. There is no known cure.
Solanezumab is one of three drugs in late-stage testing that seek to alter the course of the disease. Results on one drug were disappointing, and results of the other won't be ready until early next year.
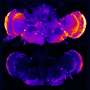
Solanezumab aims to bind to and help clear the sticky deposits that clog patients' brains. The two studies each had about 1,000 patients, about two-thirds with mild disease and one-third with moderately severe Alzheimer's, in 16 countries. Their average age was 75.
The main measures were two tests—one reflecting language, memory and thinking and the other, ability to perform daily activities such as eating and grooming. The combined results on the mild disease patients showed a nearly 2-point difference in the roughly 90-point score on thinking abilities. Previous studies suggest that a change of 3 to 4 on the score is needed to show a clinical benefit, like an improvement in how well patients can take care of themselves.
"It's a small difference," Dr. Rachelle Doody of Baylor College of Medicine said of the drug's effect. She heads a nationwide research network funded by the National Institute on Aging that did an independent analysis of Lilly's results on the studies and presented them Monday at the conference.
Still, "you slow the decline" with the drug, she said.
Independent experts cautioned that the improvement was small, and needs to be verified in another study.
"I hate to get too enthusiastic ... there's a flicker of a signal" of benefit, but less than what some other once-promising treatments showed, said Dr. Sam Gandy, head of Alzheimer's disease research at Mount Sinai School of Medicine in New York.
Dr. Ronald Petersen, director of Alzheimer's research at the Mayo Clinic, called the drug's effect "subtle" and said it may mean just that "somebody remembers one extra word out of a 15-word list" without any real improvement in how well they live.
The drug, if ever approved, is likely to be expensive, and that means "we need more evidence" of its benefit to justify its use, Petersen said.
Encouragingly, solanezumab had few side effects. About 1 percent of people on the drug had some chest pain from reduced blood flow to the heart. There were few cases of worrisome brain swelling and small bleeding in the brain, an effect that caused concern with another experimental Alzheimer's drug, bapineuzumab by Pfizer Inc. and Johnson & Johnson's Janssen Alzheimer Immunotherapy unit.
That drug failed to help patients in two late-stage studies announced last month, but did show signs of hitting its target and clearing deposits from the brain.
The Lilly drug seems to be safer, and that is an advantage, said Dr. Norman Relkin, head of a memory disorders program at New York-Presbyterian Hospital/Weill Cornell Medical Center.
"There is some cause for encouragement here. It's not the magnitude we'd like to see" but certainly warrants further studies on the drug, he said.
Relkin heads testing of the third drug in late-stage development—Gammagard, by Baxter International Inc. Results are expected early next year.
Meanwhile, Dr. Eric Siemers, senior medical director for Lilly, said the company will discuss its results and next steps with the Food and Drug Administration.
Lilly shares closed up $2.55, or 5.3 percent, to $50.78. During the day they peaked at $50.94, their highest price since April 2008. (This is also their highest closing price since that month.) The shares are up 19.8 percent since August 24.
More information:
Alzheimer's info: www.alzheimers.gov
Alzheimer's Association: www.alz.org
Copyright 2012 The Associated Press. All rights reserved. This material may not be published, broadcast, rewritten or redistributed.