October 5, 2012 report
Morphine and cocaine affect reward sensation differently
(Medical Xpress)—A new study by scientists in the US has found that the opiate morphine and the stimulant cocaine act on the reward centers in the brain in different ways, contradicting previous theories that these types of drugs acted in the same way.
Dr Ja Wook Koo of the Nestler Lab at the Mount Sinai School of Medicine in New York and colleagues, carried out mice studies on the effects of opiates and stimulants on regions of the brain known as the ventral tegmental area (VTA) and the nucleus accumbens. The team used optogenetic techniques in the research, as a similar team at the lab did in earlier research into how cocaine becomes addictive, as reported in Phys.org in 2010.
Morphine and cocaine both affect the flow of the neurotransmitter dopamine, which has been shown to be important in the feeling of reward. When a dopamine neuron is stimulated it releases dopamine, which is then taken up by neighboring cells. Any excess is reabsorbed into the original dopamine neuron by a process known as "reuptake."
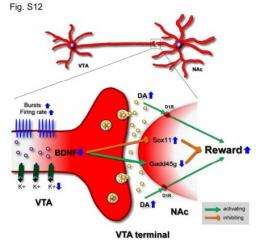
Cocaine is known to block reuptake, and the excess dopamine leads to an enhanced reward effect. Cocaine is also known to make the cells in the nucleus accumbens, which receives signals from the VTA, more sensitive to cocaine. It was already known a protein called brain-derived neurotrophic factor (BDNF) in the VTA region of the brain enhances the reward response to cocaine.
The new study shows that BDNF has the opposite effect when morphine is present, decreasing the reward response and the development of addiction rather than enhancing it. The researchers identified numerous genes regulated by BDNF and associated with its effects. They used genetic techniques to suppress BDNF, and then directly excited the neurons in the nucleus accumbens that normally receives transmitted impulses from the VTA.
They found that suppressing BDNF in the VTA allowed morphine to increase the excitability of dopamine neurons and hence enhance the reward. When they optically excited the dopamine terminals in the nucleus accumbens that normally receive the transmissions from the VTA, they also found a reversal in the normal effect of BDNF.
Knowledge of the molecular basis of the effects of drugs such as morphine and cocaine and how they can lead to addiction could lead to more effective treatments of addicts in the future. The paper was published in the journal Science this week.
The protein BDNF has also been implicated in overeating and obesity, as reported in Phys.org. This research found that suppression or deletion of BDNF led rats to overeat and become obese.
More information: BDNF Is a Negative Modulator of Morphine Action, Science 5 October 2012: Vol. 338 no. 6103 pp. 124-128 DOI: 10.1126/science.1222265
ABSTRACT
Brain-derived neurotrophic factor (BDNF) is a key positive regulator of neural plasticity, promoting, for example, the actions of stimulant drugs of abuse such as cocaine. We discovered a surprising opposite role for BDNF in countering responses to chronic morphine exposure. The suppression of BDNF in the ventral tegmental area (VTA) enhanced the ability of morphine to increase dopamine (DA) neuron excitability and promote reward. In contrast, optical stimulation of VTA DA terminals in nucleus accumbens (NAc) completely reversed the suppressive effect of BDNF on morphine reward. Furthermore, we identified numerous genes in the NAc, a major target region of VTA DA neurons, whose regulation by BDNF in the context of chronic morphine exposure mediated this counteractive function. These findings provide insight into the molecular basis of morphine-induced neuroadaptations in the brain's reward circuitry.
© 2012 Medical Xpress