Clinical trial results for BIND-014 presented at AACR 2013
The nanoparticle drug BIND-014 is effective against multiple solid tumors, according to results generated by the Translational Genomics Research Institute (TGen) and Scottsdale Healthcare, and presented today at the American Association for Cancer Research (AACR) Annual Meeting 2013.
Data for the study was generated at the Virginia G. Piper Cancer Center Clinical Trials, a partnership of TGen and Scottsdale Healthcare.
Dr. Daniel Von Hoff, TGen Physician-In-Chief and Chief Scientific Officer of Scottsdale Healthcare's Clinical Research Institute, will present A Phase 1 Study of BIND-014, a PSMA-targeted Nanoparticle Containing Docetaxel, in Patients with Refractory Solid Tumors during an AACR session at 1 p.m. EDT today at the Washington, D.C., Convention Center, Room 146.
Dr. Von Hoff, the study's Principal Investigator, will present complete Phase 1 clinical data of BIND-014, which is produced by BIND Therapeutics, a clinical-stage biopharmaceutical company developing a new class of highly selective targeted and programmable therapeutics called Accurins. BIND-014 is the company's lead drug candidate.
In 28 patients with advanced or metastatic solid tumors, BIND-014—with its targeted docetaxel Accurin—was shown to be generally safe and well-tolerated at the established maximum dose of 60 mg/m2. BIND-014 showed encouraging signs of anti-tumor activity, including one complete response, three partial responses and five patients with stable disease lasting at least four, 12-week-plus cycles. In addition, the pharmacokinetic (PK) profile of BIND-014 was substantially different from the published PK of conventional docetaxel.
"This Phase 1 trial has successfully established the safety and tolerability profile and maximum tolerated dose of BIND-014 in patients with advanced or metastatic solid tumor cancers," said Dr. Von Hoff, F.A.C.P., TGen's Distinguished Professor. "There is a critical need for targeted treatment options for patients with difficult-to-treat solid tumors, and we look forward to further evaluating the potential of BIND-014 in patients with specific solid tumor types in the near future."
"In addition to confirming the safety, tolerability and maximum tolerated dose of BIND-014, these data also provide encouraging signs of anti-tumor activity in a variety of solid tumors," said Dr. Gregory Berk, Chief Medical Officer of BIND Therapeutics. "Based on these data, BIND is moving expeditiously to advance BIND-014 into multiple Phase 2 clinical trials in 2013 including non-small cell lung cancer, prostate cancer and bladder cancer."
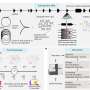
BIND-014 represents the first targeted and programmable Accurin nanomedicine to reach the clinic from BIND's proprietary drug development platform, which creates targeted therapeutics designed to accumulate at the site of disease for high drug concentration and maximum therapeutic effect. BIND-014 employs a combination of a targeted biodegradable nanoparticle and docetaxel, a well-established chemotherapy agent.
Dr. Von Hoff's presentation of BIND-014 is consistent with previously reported preliminary observations in which safety, tolerability and efficacy in multiple tumor types was demonstrated:
- BIND-014 was generally safe and well-tolerated with transient and manageable neutropenia as the dose limiting toxicity. Minimal neuropathy, mucositis, fluid retention, rash, and nail changes were observed.
- Established the maximum tolerated dose of 60 mg/m2 when administering BIND-014 on a once every 3 week (Q3W) schedule.
- Evidence of anti-tumor activity was shown with BIND-014 at 60mg/m2 in nine out of the 28 patients treated, ranging from one complete response (cervical cancer), three partial responses (non-small cell lung cancer, prostate and ampullary) and five patients with stabilization of disease lasting at least four cycles (> 12 weeks; pancreatic, colorectal, gall bladder, tonsillar and anal cancer).
- The PK profile of BIND-014, characterized by prolonged and elevated encapsulated docetaxel levels, was highly differentiated from published PK of conventional docetaxel.