Deadliest cancers may respond to new drug treatment strategy
UC San Francisco researchers have found a way to knock down cancers caused by a tumor-driving protein called "myc," paving the way for patients with myc-driven cancers to enroll in clinical trials for experimental treatments.
Myc acts somewhat like a master switch within cells to foster uncontrolled growth. Until now, it has been impossible to target with drugs.
The discovery of an unexpected biochemical link within tumor cells should lead to clinical trials for experimental drug treatments that indirectly target myc and that already are being evaluated in human studies, the researchers said.
UCSF Helen Diller Family Comprehensive Cancer Center scientists led by Davide Ruggero, PhD, and Kevan Shokat, PhD, used one such drug to stop tumor growth in a mouse model of myc-driven lymphoma and multiple myeloma types of blood cancer.
Their study is published online in Proceedings of the National Academy of Sciences (PNAS).
Previously Ineffective Drug Therapies
Unrestrained myc activity is a major player in many cancers, including cancers of the lung, colon, breast, brain, prostate and blood. Abnormal myc in cancer often is associated with poor treatment outcomes, including death.
Although other cancer-associated proteins have been successfully attacked with targeted therapies in recent years, the myc protein has continued to elude efforts to develop drugs that target it. In the PNAS paper, the UCSF researchers describe how they found a way to indirectly, but effectively, target myc-driven tumors.
The researchers discovered that cancerous myc can be thwarted by treatment that targets a specific function performed by another protein, called mTOR. The mTOR protein is part of a different biochemical pathway controlling protein production and metabolism, one that also often takes a crooked turn in tumors.
Protein Production in Cancer Is Promising Target
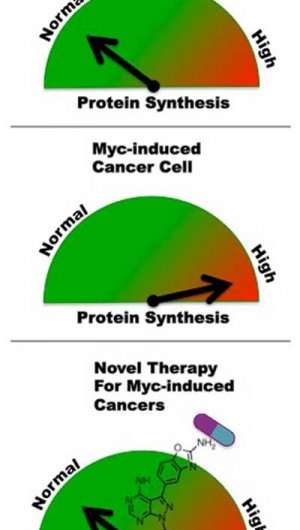
Ruggero has for several years been probing the ability of tumor cells to make extraordinary amounts of protein to sustain their rapid growth and immortality. He also explores ways to target this excess protein production in cancer.
"One of the major and immediate downstream effects of myc activation is a dramatic increase in the capacity of affected cells to make protein," Ruggero said "This, in turn, leads to increased cell survival and proliferation, and to unstable genomes that foster additional mutations that turn these abnormal cells into tumor cells."
In earlier studies, Ruggero found that myc not only drives protein production, but also that myc-driven cancer cells become absolutely dependent upon this ability to make abnormal amounts of protein. When he genetically manipulated myc-driven cancer cells to slow protein production, they committed suicide, as abnormal cells are supposed to do for the greater good.
"Tumors become addicted to excessive protein production, and mutant myc itself seems to depends on it," Ruggero said.
When present in tumors, both abnormal myc and abnormal mTOR are known to be able to rev up protein production and to foster cell growth. However, it was unclear how this myc-driven protein production could be therapeutically targeted, Ruggero said.
In the new study, the UCSF team discovered that myc relies in part on mTOR to secure its protein supply. First mTOR disables a protein that acts as a tumor suppressor, called 4EBP1. The disabling of 4EBP1 releases normal constraints on protein production within the cell. Previously, other molecular actors had been thought to play leading roles in triggering excess protein production directed by myc.
"The discovery that myc converges on the same downstream path as mTOR was surprising to us," Ruggero said.
Zeroing In on the mTOR Protein
The researchers targeted mTOR with an experimental drug based on a prototype first designed by Shokat, a chemist and an expert in designing molecules to target this type of protein, called a kinase. In the mice, drug treatment caused a shutdown of excess protein production in myc-driven cancer cells. Myc no longer was able to drive tumor growth, cancer cells committed suicide, and the treated mice survived longer.
"In the clinic, we frequently test myc levels in patients' tumors, for disease prognosis and to predict treatment response," said Michael Pourdehnad, MD, a clinical oncologist at UCSF with Ruggero's lab and the first author of the study. "Yet, the lack of specific therapies to target myc-driven cancers is frustrating. Our discovery may provide a novel solution for these patients."
"We are excited by the work of Dr. Pourdehnad and colleagues and believe these results are an important advance in understanding the role of myc pathway dysregulation in multiple myeloma, and ultimately allow for the development of therapeutic strategies to address it," said Jeffrey Wolf, MD, a UCSF blood disorder specialist and director of the Stephen and Nancy Grand Multiple Myeloma Translational Initiative at UCSF, a sponsor of the research.
The drug used in the study, called MLN0128, is made by Millennium, an independently operated subsidiary of Takeda Pharmaceutical Co., Ltd., based in Cambridge, Mass., and it is being evaluated in clinical trials to treat a variety of cancers. It had not previously been viewed as a weapon against myc-driven tumors, according to the UCSF researchers.
Currently sold drugs directed against mTOR do not inhibit its ability to target 4EBP1, which Ruggero refers to as a "master regulator" of protein production.
"This is a unique therapeutic approach to make myc druggable in the clinic," Shokat said.
Additional co-authors of the study are UCSF graduate students Morgan Truitt and Greg Ducker, and Imran Siddiqi, MD, PhD, a pathologist at the University of Southern California. Additional funds for the research were awarded by the National Institutes of Health (Grants R01CA154916 and R01 CA140456), and the Waxman Foundation. Shokat is supported by the Howard Hughes Medical Institute and Truitt is supported in part by an HHMI fellowship. The authors declare no conflict of interest in this research.
UCSF is a leading university dedicated to promoting health worldwide through advanced biomedical research, graduate-level education in the life sciences and health professions, and excellence in patient care.
More information: www.pnas.org/content/early/201 … /1310230110.abstract















