Researchers gain insight into how ion channels control heart and brain electrical activity
Virginia Commonwealth University researchers studying a special class of potassium channels known as GIRKs, which serve important functions in heart and brain tissue, have revealed how they become activated to control cellular excitability.
The findings advance the understanding of the interaction between a family of signaling proteins called G proteins, and a special type of cell membrane ion pore called G protein-sensitive, inwardly rectifying potassium (GIRK) channels. The findings may one day help researchers develop targeted drugs to treat conditions of the heart such as atrial fibrillation.
In the study, published this week in the Online First section of Science Signaling, a publication of the American Association for the Advancement of Science (AAAS), researchers used a computational approach to predict the interactions between G proteins and a GIRK channel.
Rahul Mahajan, a M.D./Ph.D. candidate in the VCU School of Medicine's Department of Physiology and Biophysics, undertook this problem for his dissertation work, under the mentorship of Diomedes E. Logothetis, Ph.D., chair of the Department of Physiology and Biophysics and the John D. Bower Endowed Chair in Physiology in the VCU School of Medicine. They developed a model and tested its predictions in cells, demonstrating how G proteins cause activation of GIRKs.
"Malfunctions of GIRK channels have been implicated in chronic atrial fibrillation, as well as in drug abuse and addiction," said Logothetis, who is an internationally recognized leader in the study of ion channels and cell signaling mechanisms.
"Understanding the structural mechanism of G?? activation of GIRK channels could lead to rational based drug design efforts to combat chronic atrial fibrillation."
In chronic atrial fibrillation, the GIRK channel is believed to be inappropriately open. According to Logothetis, if researchers are able to target only the specific site that keeps the channel inappropriately open, then any unrelated channels could be left unaltered, thus avoiding unwanted side effects.
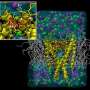
Crystal structures of GIRK channels, which preceded the current study, have revealed two constrictions of the ion permeation pathway that researchers call "gates": one at the inner leaflet of the membrane bilayer and the other close by in the cytosol, which is the liquid found inside cells.
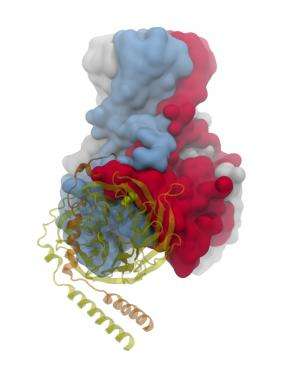
"The structure of the G?? -GIRK1 complex reveals that G?? inserts a part of it in a cleft formed by two cytosolic loops of two adjacent channel subunits," Logothetis said. "This is also the place where alcohols bind to activate the channel. One can think of this cleft as a clam that has its shells either open or shut closed. Stabilization of this cleft in the 'open' position stabilizes the cytosolic gate in the open state."
GIRKs are activated when they interact with G proteins coupled to receptors bound to stimulatory hormones or neurotransmitters. In heart tissue, acetylcholine released by the vagus nerve activates these channels, which hyperpolarize the membrane potential and slow heart rate. In brain tissue, GIRKs inhibit excitation by acting at postsynaptic cells.
G proteins are composed of three subunits, a, b, and g. Since 1987, researchers have known that the Gbg subunits directly activate the atrial GIRK channel, but an atomic resolution picture of how the two proteins interact remained elusive until now.
Moving forward, the team would like to use computational and experimental approaches to build and test the structures of the rest of the components of the G protein complex – for example, the Ga subunits and the G protein-coupled receptor – around the G??-channel complex, which is the structure the team has already achieved.
The study is titled: "A Computational Model Predicts the Action of G??; at an Inter-Subunit Cleft to Activate GIRK1 Channels."
More information: stke.sciencemag.org/cgi/conten … /sigtrans;6/288/ra69