A new target for diabetes treatment

In her synthetic biology lab, Karmella Haynes focuses much of her effort on developing better ways of exploring how human body cells work – or don't work like they should. She'll be applying her expertise in that area to a major new research endeavor to produce more effective treatments for diabetes.
The project is being undertaken by the national Synthetic Biology Engineering Research Center (SynBERC), which is supported by the National Science Foundation (NSF). SynBERC members include the Massachusetts Institute of Technology (MIT), Harvard Medical School and Stanford University.
Haynes became an affiliate researcher for the center as a result of the synthetic biology research she conducted at Harvard before joining Arizona State University, where she is an assistant professor in the School of Biological and Health Systems Engineering, one of ASU's Ira A. Fulton Schools of Engineering.
The NSF recently awarded her an $81,000 grant to support her role in the project. She will seek to develop more advanced synthetic monitors to track the development and health conditions of body cells.
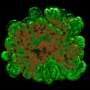
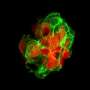
In this case, Haynes will zero in on pancreatic islets cells – tiny clumps of cells in the pancreas that produce glucagon and insulin, which helps the body burn sugar and maintain blood sugar levels.
"When something goes wrong within these clumps of cells, you develop diseases such as diabetes," she explains.
Diabetes is a major disabling and deadly disease that is projected to afflict about 300 million people by 2025, according to the World Health Organization.
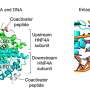
Glucagon is a peptide hormone that the pancreas secretes. It raises blood glucose levels – the opposite of the function of insulin, which lowers blood glucose levels.
Haynes will grow pancreatic cells that produce glucagon and insulin in an artificial culture (outside the body), enabling her to study the effects of particular synthetic proteins and DNA on those cells.
In the past, such cell cultures have been grown on flat surfaces. Haynes will collaborate with labs at MIT to grow and study the cells in a three-dimensional translucent biomaterial.
Being able to make observations from a three-dimensional view "gives us a much more holistic view of how treatments affect the pancreatic islet tissue, and of ways we can genetically manipulate cells so a disease can be treated effectively," she explains. "We can gain a deeper understanding of the proper growth process of that small mass of pancreatic cells that is critical in preventing development of diabetes."
The cells themselves will be implanted with synthetic monitors capable of tracking changes in the chromosomal structure of the cells. That will give Haynes a precise look at various stages of cell development.
She will be designing the synthetic proteins that act as monitors, revealing "the molecular changes that occur in the cells as they grow" and allowing her "to manipulate the states of the cells to maintain healthy pancreatic tissue."
The DNA-based monitoring device signals what it finds by glowing – enabled by the use of a genetically encoded fluorescent protein that the monitor can produce.
"We can engineer the DNA-based monitor so that when the chromosome structure of the cells changes, the monitor is tripped and starts producing a green fluorescent protein," Haynes explains. "Certain changes in chromosome structure can promote cell viability or cause cell death."
The process enables close tracking of the condition of the coiled material in the chromosomal structure of pancreatic cells. If the coils become too loose or too condensed, they can interfere with insulin production or other proper functions of the pancreas.
Haynes' says one of her project's goals is "revealing the physics underlying the coiling and uncoiling of these strands of DNA" and using that knowledge to "design small proteins that go into cells and actually control the coiling and uncoiling of DNA so that the pancreas islets have plenty of cells that are producing proper levels of insulin."
A portion of her NSF grant will support students in getting hands-on lab experience. One undergraduate and one graduate student will be part of Haynes' SynBERC project research team.