November 26, 2013 report
Mapping the entire brain with new and improved Brainbow II technology
(Medical Xpress)—Among the many great talks at the recent annual meeting of the Society for Neuroscience were three special lectures given sequentially during the evenings. The first described how we might translate the known circuit diagram of the worm, and the range of neural activities it supports, into it's play in a 2D world. The second followed with how we might trace the trickle of information from the larger 3D world, through the more complex theater of the fly brain, and back out again. The third, and most gripping story in the trilogy, was Jeff Lichtman's talk about using his new technology—known as Brainbow II— to turn the wild synaptic jungle into a tame and completely taxonomized arboretum which we can browse at our leisure.
A movie of a millimeter-sized worm learning to recognize and wriggle free from a mini-lariat may not be the critics choice. However, considering that the critical neurons and synapses involved in this particular behavior can now be genetically isolated, and watched in detail, many neurobiologists are fairly excited. We still don't have whole-brain electrical activity maps for the 302 neurons (and 50 glial cells) in this creature, or even high resolution calcium clips of these cells—but that may not be required. Many neurons do not bother to use discrete spikes when they are only sending signals across short distances, and sometimes they don't even bother to build axons.
In this case, if we want to understand how the worm acquires the lariat escape trick, perhaps we might instead just watch its mitochondria as their host neurons stir in seeming alarm. Indeed if we were to watch nothing but mitochondria, most of what we might learn about a given neuron through the use of a whole host of other imaging technologies, is already contained within their dynamics. One could probably infer not just the membraneous outlines of a neuron by watching the limits of mitochondrial excursions, but also infer the changes in the shape of the individual neurites. Further in this vein, we also now appreciate that mitochondria don't just respond to the calcium flows mentioned above, they are in fact calcium-controlling organelles by trade.
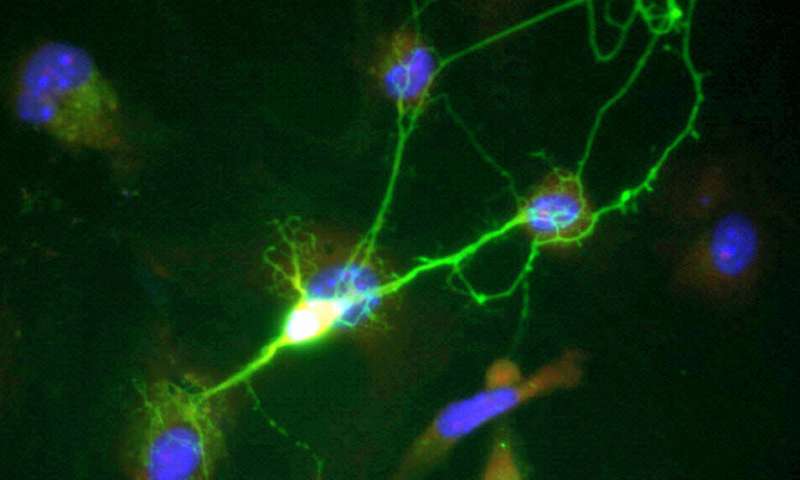
One thing that we learned from Brainbow I, which was further highlighted with the expanded palette of Brainbow II, is that labeling everything can be as bad as labeling nothing at all. Part of Brainbow II's feature set, is more control for the selective labeling of synapses from different kinds of interneurons, and also the processes of glial cells. In order to reap the benefits of Brainbow II technology and create detailed computer reconstructed images of these cells, Lichtman's group had to build high speed brain slicing and processing instruments, as well as high power electron microscopes to create the images.
Lichtman reported that together with Zeiss, a new high-throughput 61-beam scanning electron microscope is currently under development. This massive device does not look like something that could just be slid into an elevator and sent to a fourth-floor lab. I asked @zeiss_optics about pricing and availability on this behemoth, along with focused ion beam attachment, and they said that they are offering a nice rebate on orders of two or more. Even still, the result of many months of protected effort has thus far only yielded the structure of just a small piece of brain.
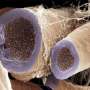
But what a structure it is. The crowning achievement, shown at the convention was distilled into a cylindrical EM reconstruction of a piece of mouse brain smaller than a grain of sand. In the center of this volume was the proximal shaft of a pyramidal cell apical dendrite surrounded by all manner of synaptic elements. If you were ever confounded by the famous 4-color mapping thereom, then Brainbow-style synapse tracing may not be for you. In this volume there are around 680 nerve fibers that can be resolved, together with 774 synapses. A key finding by Lichtman is that mere contact alone, does not a synapse make. By tracking perfectly resolved synaptic vesicles, he was able to show that of every ten plausible synaptic options, perhaps only one or two neighboring profiles turned out to be an actual synapse.
The final point Lichtman made is that now that it is possible to extract the complete membrane topology, including organelles, of an arbitrary region of the brain, formerly unimagined questions might be posed and answered with the click of a mouse. The question he alluded to is the one I raised above, namely, how are the mitochondria distributed, and what are they doing? While this is in large part, a question for live, video microscopy, much can be learned about the state of a given synapse just prior to being fixed by it's mitochondria. Similarly, much might be also be inferred about the next plausible state of the neural geometry under consideration, provided one knows what to look for.
The one finding here that Lichtman mentioned was that axons have relatively small mitochondria compared to those in the body and dendrites. That may be a seemingly sterile finding when considered alone. But that same afternoon at the conference, there was an exciting talk describing how certain mitochondria are extravasated, or expelled, by axons in the visual system. They are then taken up by astrocytes for processing—a rather surprising finding. It has been known that in some organs mitochondria can be exchanged between cells, much to the benefit of the recipient cell, though for neurons, this is the first report of such phenomena. I did look later at the literature, and this fractionation of mitochondria by size in the polar elements of neurons has actually been known for some time, leading one to guess what other potential findings the Lichtman group might actually possess.
What Lichtman presented is really not a connectome, or a "netlist" of circuit board connections, per say. To date, nobody has even put force a reasonable transform to derive a connectome from a given 3D membrane mesh topology, or even of what use it would be if we had one. Meanwhile, attempts to model the fissions, fusions, and general ramblings of the mitochondria as a function of their genetic makeup, and the positions they take up inside the cell, have already begun. If genetically questionable mitochondria with expired membrane potentials tend to be degraded by fusion with lysosomes near the nucleus, we might ask, can they be blamed for pumping out axons and transporting themselves as far away as possible—even out of the cell entirely?
Clearly, anthropomorphizing mere motile sacks of DNA and enzymes is not the only tool we have to hack the brain. But insofar as the brain is just a complex system of microscopic tubes, it may make sense to take a closer look at the creatures that build and maintain them. In this light, the science of connectomes becomes the science of mitochondria, the mitochondriome perhaps. As much as we can better understand the collective activity of the brain through the remembrance of neurons as once-feral protists now encased in the skull, our understanding of neurons is enhanced by recalling their mitochondria as once-free bacteria now largely trapped in them.
More information: New Tools Light Up the Intricacies of the Brain, Science 22 November 2013: Vol. 342 no. 6161 pp. 917-918. DOI: 10.1126/science.342.6161.917
Abstract
Europe and the United States have recently launched large neuroscience research initiatives aimed at untangling the complex workings of the human brain. Meeting this aim will require new tools that can map the brain's structure from the level of individual synapses to entire neural circuits and measure the activity of many neurons at once. At the Society for Neuroscience meeting in San Diego last week, researchers presented new technologies that could help achieve those goals and discussed approaches for dealing with the tidal wave of data that such tools are already producing. Highlights from the meeting include advances in automated, high-throughput electron microscopy of brain tissue and a new imaging technique that can track the activity of up to 1000 neurons in real time as animals learn.
© 2013 Medical Xpress