FDA approves implanted brain stimulator for epilepsy
(HealthDay)—The U.S. Food and Drug Administration on Thursday gave its approval to a new implanted device that lowers the rate of seizures among people with epilepsy.
"The neurostimulator detects abnormal electrical activity in the brain and responds by delivering electrical stimulation intended to normalize brain activity before the patient experiences seizure symptoms," Christy Foreman, director of the Office of Device Evaluation in the FDA's Center for Devices and Radiological Health, said in an agency news release.
Kelly O'Brien, 28, has epilepsy and said the device—called the RNS Stimulator—has been life-changing.
"It has given me an independence I did not have before," said O'Brien, who lives in Columbus, Ohio. "Since getting the device, my seizures have stopped and I am doing things I was not able to do in the past. The biggest thing is, I'm now able to drive again."
Smaller and thinner than an implantable heart defibrillator, the battery-powered, programmable device is placed just under the skull during surgery. Electrodes reach from the device to the one or two places in the patient's brain that create the abnormal electrical activity that causes seizures. The RNS System, made by California-based company NeuroPace, works by short-circuiting nerve cells in that area to normalize brain activity before a seizure is triggered.
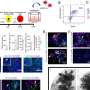
The FDA decision follows a unanimous vote in February in favor of the device by an FDA panel that assessed its effectiveness and safety. According to the FDA, the new pre-marketing approval granted to the RNS System was based on a three month clinical trial involving 191 patients whose epilepsy had not responded to drug therapy.
The study found that patients with a switched-on device saw their rate of monthly seizures fall by an average 38 percent, compared to a 17 percent reduction among patients who had received the implant but had had it switched off. For some patients, the device worked especially well, according to the FDA—29 percent of patients with a switched-on device saw the number of seizures they experienced per month fall by half.
Reductions in seizure frequency linked to the RNS System continued over a 2-year follow-up period, the agency added.
"These are patients who have no other resort for treatment of their epilepsy, and this device offers new hope for them," said Dr. Dileep Nair, an epileptologist and Section Head of the Cleveland Clinic's Epilepsy Center. The center was one of 15 sites in the United States to participate in clinical trials.
"We badly need new, effective therapies for the hundreds of thousands of people in this country as well as the millions around the world who live with uncontrolled seizures," Warren Lammert, chairman of the patient advocacy group the Epilepsy Foundation, said in a Neuropace news release.
He said the new device "integrates the best of technology and neurology, and is an important new treatment option for these individuals and their families."
Dr. Orrin Devinsky is director of the NYU Comprehensive Epilepsy Center in New York City. He said the center has been involved in the RNS Stimulator trials, "and have seen significant clinical improvements, including patients who are now seizure-free and driving for the first time in their life."
The device is designed specifically for people 18 and older with partial-onset epilepsy, which occurs when one or more fixed locations in a person's brain start the cascade of nerve firing that creates a seizure.
NeuroPace has also done two studies involving a total of 256 patients who were monitored for a period of between two and nine years, without any significant problems, according to Neuropace president and CEO Frank Fischer.
Epilepsy is a brain disorder in which a person suffers repeated seizures over time. It affects more than 2 million Americans, according to the Epilepsy Foundation, making it the third most common neurological disorder in the United States, after Alzheimer's and stroke. Seizures are episodes of disturbed brain activity that cause changes in attention or behavior. Brain cells keep firing instead of acting in an organized way. The malfunctioning electrical system of the brain causes surges of energy that can cause a person to have muscle contractions or to black out.
Physicians can modify the programming of the device even after it has been implanted, to reflect a patient's needs over time, Fischer said. They can also observe the brain activity of a patient from a laptop computer in their office—to help them manage a patient's treatment, he said.
Speaking in February at the time of the FDA panel's approval, Fischer said it was too soon to say what the device might cost. However, comparable systems for heart problems range in price from $30,000 to $35,000, not including the cost of the surgery to implant the device. The battery that powers the epilepsy device lasts about three years. When it fails, a new device has to be substituted in a 30-to-60-minute outpatient surgical procedure, Fischer said.
The FDA did note some important safety issues with the RNS System. Users cannot undergo MRI procedures, or other procedures such as diathermy (electrically induced heat), electroconvulsive ("electroshock") therapy or transcranial magnetic stimulation. "The energy created from these procedures can be sent through the neurostimulator and cause permanent brain damage, even if the device is turned off," the FDA explained.
Health issues that could also occur include infections at the site of the implant and premature battery depletion, the FDA said.
More information: For more on epilepsy, visit the Epilepsy Foundation .
Copyright © 2013 HealthDay. All rights reserved.