Disruptions in a protein-processing pathway appear to promote aggressive proliferation and invasion in tumor cells
Many proteins at the cell surface are 'decorated' with carbohydrate molecules called glycans. Changes in a cell's glycan profile can greatly alter how it behaves and interacts with its environment, and abnormalities in this process are a common feature of cancer cells. Researchers led by Frederic Bard of the A*STAR Institute of Molecular and Cell Biology in Singapore have now uncovered a potentially critical link between the glycan addition process and invasive tumor growth.
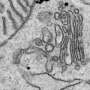
Cancer cells often produce abnormally high levels of proteins modified with a glycan precursor known as Tn. In healthy cells, newly translated proteins are processed in a structure called the endoplasmic reticulum (ER) before moving on to the Golgi apparatus. Within the Golgi, proteins receive the Tn modification from enzymes known as GalNAc-Ts, and Tn is subsequently transformed into more complex glycans by other Golgi enzymes. Scientists have long assumed that cancer cells experience defects in this latter pathway, which ultimately directs excess Tn-modified proteins to the cell surface.
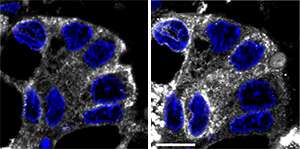
Bard and David Gill, however, arrived at an alternative hypothesis. In previous research, they determined that Src, a signaling protein overactive in many cancers, can cause GalNAc-Ts to relocate from the Golgi to the ER. They subsequently determined that the presence of GalNAc-Ts in the ER is sufficient to induce a sharp elevation in Tn levels. Contrary to expectations, Tn in cultured cells was largely retained within the ER rather than being transported to the cell surface. The researchers observed similar behavior in a diverse array of hundreds of tumor samples (see image).
When Bard and co-workers examined tumor cell lines that had been genetically modified to forcibly shift GalNAc-T localization to the ER, they observed that this made the cells far more aggressive and prone to invasive growth. "The most surprising finding was how readily ER glycosylation stimulates cell migration and cell adhesion," says Bard. "It seems counterintuitive that modifications occurring inside the ER would produce dramatic changes in how the cell interacts with its environment."
Bard and his co-workers hypothesize that these effects are driven by proteins that escape directly to the cell surface after undergoing Tn modification in the ER. By exploring this question in future work, Bard aims to uncover vulnerabilities that could be exploited to disrupt the tumor growth machinery. "We expect that this Src-mediated pathway is activated in 60 to 80 per cent of human tumors and so any benefits could apply to a lot of patients," he says.
More information: Gill, D. J., Tham, K. M., Chia, J., Wang, S. C., Steentoft, C. et al. Initiation of GalNAc-type O-glycosylation in the endoplasmic reticulum promotes cancer cell invasiveness. Proceedings of the National Academy of Sciences USA 110, E3152–E3161 (2013). dx.doi.org/%20%20%20%2010.1073/pnas.1305269110
Gill, D. J., Chia, J., Senewiratne, J. & Bard, F. Regulation of O-glycosylation through Golgi-to-ER relocation of initiation enzymes. The Journal of Cell Biology 189, 843–858 (2010). dx.doi.org/10.1083/jcb.201003055