New non-surgical treatment for common, vexing eye condition
A new report reveals a potential breakthrough in the treatment of a common eye ailment known as pterygium (Surfer's eye) that impacts the vision, eye health, and cosmetic appearance of countless sufferers.
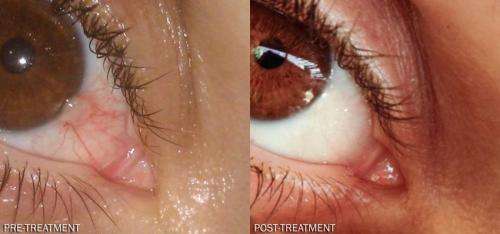
The newly published report shows that eye drops containing the anti-anginal drug dipyridamole (Persantin, Cardoxin) led to almost total disappearance of an inflamed pterygium in a 35 year old otherwise healthy woman.
Dipyridamole is a drug in use over the past 55 years to treat other disorders, but now found to have this remarkable new use.
Pterygium is a disorder in which a non-cancerous growth develops on the white conjunctiva of the eye and over time invades the cornea. In some countries it affects up to 25% of the population. As the growth spreads, patients can develop vision problems as well as significant discomfort from complications such as dry eye, inflammation, irritation, and foreign body sensation. Additionally, because of their location in the eyes, pterygia are a cause of substantial cosmetic concern for sufferers.
Until now, eye surgery has been the only curative option, aided by medications trying to lessen the disorder's symptoms. However, even after eye surgery, pterygia often recur.
The new report's lead author, Moshe Rogosnitzky, who is Co-Founder and Director of Research at the MedInsight Research Institute, discovered that administration of dipyridamole eye drops significantly reduced a pterygium and completely resolved the associated inflammation and other symptoms.
Clinical trials are now being plannedfor pterygia, pingueculae, and other common eye disorders and their complications such as dry eye and inflammation.
One particular advantage to this discovery is that dipyridamole is a widely-approved anti-thrombosis medication that has been in use for over 55 years. Its safety profile is well-established; as such, fast-track development of dipyridamole eye drops as a repurposed drug is feasible.
Moshe Rogosnitzky commented on this finding, "Pterygium and dry eye are debilitating disorders for which new safe solutions are urgently needed, and I believe dipyridamole has the potential to provide relief to sufferers of these intractable conditions.
Rogosnitzky, who specializes in finding new uses for old drugs, continued, "This is yet another example of the advantages of drug repurposing. Whereas bringing a new drug to market can take up to 17 years or more, finding a new use for an old drug with an excellent safety profile can lead to approval and availability in as little as two years."
More information: The findings of this report and photos of the treated eye are published in Case Reports in Ophthalmology (2014; 5:pp. 98-103) on March 25, 2014.