Newly approved brain stimulator offers hope for individuals with uncontrolled epilepsy
A recently FDA-approved device has been shown to reduce seizures in patients with medication-resistant epilepsy by as much as 50 percent. When coupled with an innovative electrode placement planning system developed by physicians at Rush, the device facilitated the complete elimination of seizures in nearly half of the implanted Rush patients enrolled in the decade-long clinical trials.
That's good news for a large portion of the nearly 400,000 people in the U.S. living with epilepsy whose seizures can't be controlled with medications and who are not candidates for brain surgery.
Epilepsy is a chronic neurological condition characterized by recurrent seizures that disrupt the senses, or can involve short periods of unconsciousness or convulsions. "Many people with epilepsy have scores of unpredictable seizures every day that make it impossible for them to drive, work or even get a good night's sleep," said Dr. Marvin Rossi, co-principal investigator of the NeuroPace Pivotal Clinical Trial and assistant professor of neurology at the Rush Epilepsy Center.
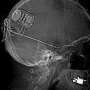
The NeuroPace RNS System uses responsive, or 'on-demand' direct stimulation to detect abnormal electrical activity in the brain and deliver small amounts of electrical stimulation to suppress seizures before they begin.
The device is surgically placed underneath the scalp within the skull and connected to electrodes that are strategically placed within the brain where the seizures originate (called the seizure focus). A programmed computer chip in the skull communicates with the system to record data and to help regulate responsive stimulation.
The unique electrode placement planning modeling system developed at Rush uses a computer-intensive mapping system that facilitates surgical placement of electrodes at the precise location in the brain's temporal lobe circuitry. When stimulated, these extensive epileptic circuits are calmed.
The modeling system predicts where in the brain the activity begins and spreads, so that the device can better influence the maximal extent of the epileptic pathway.
The device also acts as an implanted EEG for recording brain activity. This function was first shown at Rush to help determine whether the patient will further benefit from a surgical resection, in which surgeons remove a portion of the temporal lobe network.
Dr. Richard Byrne, chairman of Neurosurgery at Rush, implants the electrodes in the temporal lobes. As a result, physicians at Rush can offer patients the new implantable neurostimulator device, a surgical resection or both with the possibility of completely eliminating seizures. "This device is also being used at Rush as a foundation and inspiration for building cutting-edge hybrid stimulation therapy-drug molecule delivery systems," said Rossi.
"Devices that treat epilepsy may offer new hope to patients when medication is ineffective and resection is not an option," said Rossi. "Not long ago, it was highly unlikely that these patients would ever be free of their seizures. Now, several of our Rush patients with this device are actually able to drive, lower or even eliminate their medications and aren't as limited as they once were. There is no doubt that quality of life of the majority of our implanted patients is significantly improved."
According to the Centers for Disease Control and Prevention, in 2010, epilepsy affected approximately 2.3 million adults in the U.S. and 467,711 children under the age of 17.