Spectrum Health among first to implant neurostimulator for epilepsy

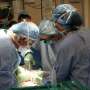
Spectrum Health is the first health system in Michigan and among the first in the nation to successfully implant a recently FDA-approved device that uses electric stimulation of the brain to treat adult epilepsy patients whose seizures have not responded to medication.
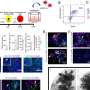
The NeuroPace Responsive Neurostimulation (RNS) System is an implantable therapeutic device designed to detect abnormal electrical activity in the brain and respond by delivering imperceptible electrical stimulation to normalize brain activity before an individual experiences seizures.
The procedure was successfully carried out May 6 in a six-hour surgery at Spectrum Health Butterworth Hospital in Grand Rapids. The first patient, Amy Owen, 44, of Marshall, Mich., is recovering at home. A second patient, Kelly Cromer, 43, of Dowagiac, Mich., underwent surgery May 14 and also is recovering at home. (Links to surgery and interview footage of Kelly Cromer).
The operations were carried out by a team led by Kost Elisevich, MD, PhD, neurosurgeon, co-chair department of clinical neurosciences, chief, division of neurosurgery, Spectrum Health Medical Group. Dr. Elisevich was assisted in the May 6 surgery by neurosurgeon Sanjay Patra, MD, and in the May 14 procedure by neurosurgeon Artur Szymczak, MD.
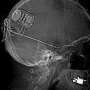
The surgery places the NeuroPace RNS Neurostimulator, essentially a battery powered microprocessor, into a cavity created in the patient's cranium. This device is connected to electrodes implanted in the brain at the site where seizures are believed to originate. The May 6 procedure was the first of its kind in the state of Michigan and was among the first 20 such procedures in the nation. Spectrum Health is one of only 10 Level 4 epilepsy centers currently approved to conduct the surgery.
"Spectrum Health's neuroscience program continues to expand its clinical and surgical offerings, providing patients highly specialized care in their region and exceeding important national quality standards," said Kevin Splaine, president, Spectrum Health Hospitals, Grand Rapids. "Our epilepsy team is specially designated for its superior medical and surgical care."
Owen was admitted to the Epilepsy Monitoring Unit at Spectrum Health Butterworth Hospital in late April. In an initial procedure, Dr. Elisevich surgically implanted electrodes into her brain to initiate the monitoring of seizures with a continuous inpatient video electroencephalographic (vEEG).
This mapping pinpointed the origin of Owen's seizures in the left temporal lobe, enabling physicians to target the optimal location for placement of the NeuroPace RNS Neurostimulator, a titanium microprocessor measuring about one by two inches, which is connected to electrodes implanted at the site of seizure origin. The monitoring also indicated that Owen was undergoing near continuous seizure activity while she slept.
While her doctors consider the operation to be a success, they caution that the work of monitoring and interrupting seizure activity in Owen's brain is only just beginning.
"Seizure activity can often be temporarily disrupted by the placing of electrodes during surgery," Dr. Elisevich said. "The next phase is to wait for her brain to start misbehaving again so that the computer can be programmed to interrupt the dysfunctional signaling."
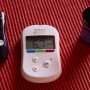
During this phase of the process, the patient uses an electronic wand to download data from the device on a daily basis. This information enables her medical team to track her seizure activity and disrupt it by programming the device to deliver short, imperceptible electrical pulses to the brain through the implanted electrodes.
"This information will enable us to identify a distinct pattern of seizure onset, which we can break down by frequency, amplitude and a number of other factors," said Owen's neurologist, Brien Smith, MD, co-chair, department of clinical neurosciences, chief, division of neurology, Spectrum Health Medical Group. "This makes us very optimistic that we can alter abnormal brain activity and significantly reduce seizure initiation and spread."
The RNS System is designed for use in combination with other therapies in reducing the frequency of seizures in individuals 18 years of age or older. It is indicated for use in patients with partial onset seizures – seizures that originate and remain in a limited area of the brain. To be eligible for the procedure, patients must have frequent and disabling seizures (motor partial seizures, complex partial seizures and/or secondarily generalized seizures), whose origin is limited to two or fewer locations in the brain.
The U.S. Food and Drug Administration (FDA) granted premarket approval for the RNS System on November 14, 2013. While employed at Henry Ford Health System, Drs. Elisevich and Smith were one of the first clinical teams in the nation to participate in clinical trials using the RNS System.
Epilepsy is the third most common neurological disorder in the United States after Alzheimer's disease and stroke, affecting 2.2 million Americans, according to the Centers for Disease Control and Prevention (CDC). Older adults are among the fastest-growing segments of the population for new cases of the disease.
Treatment with the RNS System is taking place in Level 4 epilepsy centers throughout the nation following site qualification and physician training. Spectrum Health is the first epilepsy program in West Michigan to receive a Level 4 designation by the National Association of Epilepsy Centers (NAEC). Level 4 centers have the professional expertise and facilities to provide the highest level of medical and surgical epilepsy evaluation and treatment for patients with epilepsy.