RNA combination therapy for lung cancer offers promise for personalized medicine
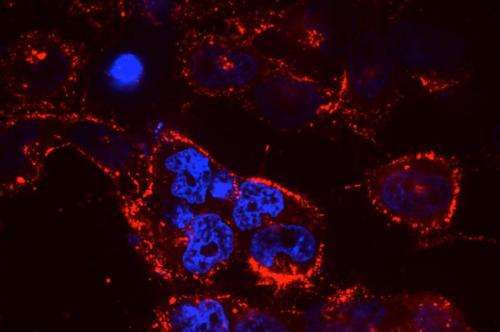
Small RNA molecules, including microRNAs (miRNAs) and small interfering RNAs (siRNAs), offer tremendous potential as new therapeutic agents to inhibit cancer-cell growth. However, delivering these small RNAs to solid tumors remains a significant challenge, as the RNAs must target the correct cells and avoid being broken down by enzymes in the body. To date, most work in this area has focused on delivery to the liver, where targeting is relatively straightforward.
This week in the journal Proceedings of the National Academy of Sciences, researchers at the Koch Institute for Integrative Cancer Research at MIT report that they have successfully delivered small RNA therapies in a clinically relevant mouse model of lung cancer to slow and shrink tumor growth. Their research offers promise for personalized RNA combination therapies to improve therapeutic response.
Delivering combination therapies
Using the "KP" mouse model, in which a mutant form of the oncogene KRAS is activated and tumor-suppressor gene p53 is deleted, researchers injected mice with RNA-carrying nanoparticles. This mouse model reflects many of the hallmarks of human lung cancer and is often used in preclinical trials. It was originally developed in the laboratory of Koch Institute Director Tyler Jacks, the David H. Koch Professor of Biology, who is co-senior author of this paper.
The nanoparticles are made of a small polymer lipid conjugate; unlike liver-targeting nanoparticles, these preferentially target the lung, and are well-tolerated in the body. They were developed in the laboratories of co-senior author Daniel G. Anderson, the Samuel A. Goldblith Associate Professor of Chemical Engineering, an affiliate of MIT's Institute of Medical Engineering and Science; and author Robert Langer, the David H. Koch Institute Professor.
In this study, researchers tested the nanoparticle-delivery system with different payloads of therapeutic RNA. They found that delivery of miR-34a, a p53-regulated miRNA, slowed tumor growth, as did delivery of siKRAS, a KRAS-targeting siRNA. Next, researchers treated mice with both miR-34a and siKRAS in the same nanoparticle. Instead of just slowing tumor growth, this combination therapy caused tumors to regress and shrink to about 50 percent of their original size.
Researchers then compared mouse survival time among four treatment options: no treatment; treatment with cisplatin, a small-molecule, standard-care chemotherapy drug; treatment with nanoparticles carrying both miR-34a and siKRAS; and treatment with both cisplatin and the nanoparticles. They found that the nanoparticle treatment extended life just as well as the cisplatin treatment, and furthermore, that the combination therapy of the nanoparticles and cisplatin together extended life by about an additional 25 percent.
Potential for personalized cancer treatments
This early example of RNA combination therapy demonstrates the potential of developing personalized cancer treatments. With efficient delivery of therapeutic RNA, any individual small RNA or combination of RNAs could be deployed to regulate the genetic mutations underlying a given patient's cancer. Furthermore, these RNA therapies could be combined with more traditional drug therapies for an enhanced effect.
"Small-RNA therapy holds great promise for cancer," Jacks says. "It is widely appreciated that the major hurdle in this field is efficient delivery to solid tumors outside of the liver, and this work goes a long way in showing that this is achievable."
"RNA therapies are very flexible and have a lot of potential, because you can design them to treat any type of disease by modifying gene expression very specifically," says James Dahlman, a graduate student in Anderson's and Langer's laboratories who, along with senior postdoc Wen Xue of Jacks' laboratory, is co-first author of the paper. "We took the best mouse model for lung cancer we could find, we found the best nanoparticle we could use, and for one of the first times, we demonstrate targeted RNA combination therapy in a clinically relevant model of lung cancer."
This investigation typifies the Koch Institute's model of bringing biologists and engineers together to engage in interdisciplinary cancer research.
"This study is a terrific example of the potential of new RNA therapies to treat disease that was done in a highly collaborative way between biologists and engineers," Langer says. "It's an example of what makes the Koch Institute very special."
Contributors to this research from Langer's and Anderson's laboratories include postdocs Omar Khan and Gaurav Sahay, former postdoc Avi Schroeder, and Apeksha Dave '13. Contributors from Jacks' laboratory include postdoc Tuomas Tammela, Sabina Sood '13, MIT junior Gillian Yang, and former research technicians Wenxin Cai and Leilani Chirino.
This story is republished courtesy of MIT News (web.mit.edu/newsoffice/), a popular site that covers news about MIT research, innovation and teaching.