May 5, 2015 feature
Brain in a bottle: A new culture medium for growing and testing neuronal cells in vitro
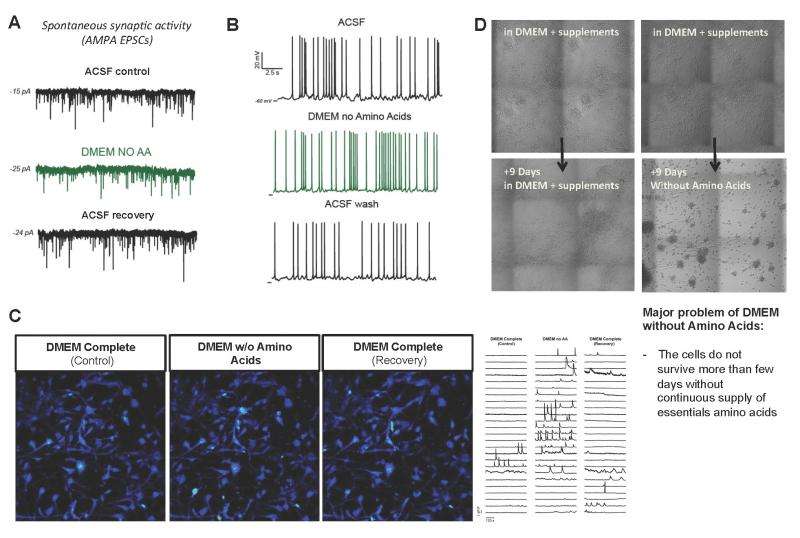
(Phys.org)—In vivo neural electrical activity is the essence of nervous system function, controlling sensory modalities, emotion, memory, behavior, and basic survival functions. Therefore, to study neurons in the laboratory it is important that in vitro neuronal models also support such electrical activity to reflect fundamental brain functions – and most human neuronal cultures are currently grown in vitro using the classic culture media DMEM (Dulbecco's Modified Eagle Medium), Neurobasal, or a mixture of the two. In contrast, laboratory experiments employing electrophysiological techniques – such as patch clamping (which allows the study of single or multiple ion channels in cells), calcium imaging – on brain slices or in culture are performed in a medium of artificial cerebrospinal fluid (aCSF).
Recently, scientists at the Salk Institute for Biological Studies, Sanford Consortium for Regenerative Medicine, La Jolla, CA, employing induced pluripotent stem cells (iPSCs) to model human neurological diseases in vitro used electrophysiology techniques to test DMEM and Neurobasal to determine their influence on fundamental neuronal activity. Surprisingly, the scientists discovered that, even though these are classic culture media, they strongly altered many crucial neurophysiological properties. Before deciding to design a new medium, the researchers tested all commercially available basal media that might be used for neuronal tissue culture; none of them supported electrophysiological activity as well as aCSF. However, even with the addition of various supplements, aCSF was not sufficient to maintain cell cultures for more than a day or two. The scientists therefore embarked on the challenging process of designing a new medium more adapted to supporting neuronal function, eventually producing BrainPhys, a novel medium that improves the differentiation and electrophysiological activity of neurons, supports long-term in vitro culture, mimics physiological conditions of the living brain, and allows for assessment of electrophysiological activity.
Dr. Cedric Bardy discussed the study and the resulting paper that he and his colleagues published in Proceedings of the National Academy of Sciences. "We reasoned that, to truly assess the physiology of neuronal cultures, it makes more sense to perform electrophysiological experiments in the same medium in which the cells were grown and maintained in the incubator," Bardy tells Medical Xpress. "At the time, we had absolutely no idea that classic culture media impaired neuronal activity. We started trying to test the electrophysiological properties of human neuronal cells in classic tissue culture media with patch clamping techniques, and while we could record some action potentials in these media, synaptic activity was totally silent."
Nevertheless, before the researchers started to question the medium, they assumed that the problem might be due to either the human neurons or the new patch clamping equipment they had just built. They quickly ruled out these hypotheses by patch clamping mouse brain slices used aCSF instead of culture medium, finding that these experiments were very successful in recording active. The next day," Bardy recalls, "we went back to patch human neurons. We started the recordings in classic culture medium, and again we could record some poor action potential activity but no synaptic activity at all. This time, while patching that same cell, we switched the extracellular solution to aCSF. It dramatically improved neuronal function, which we then confirmed by calcium imaging. I could barely believe it! We were shocked to discover that we had been culturing neurons for decades in media containing neuroactive components that dramatically impair such basic and important neuronal functions." Moreover, he adds, it is important– at least in most cases – that tissue culture models recapitulate in vivo conditions as realistically as possible. Since synaptic and electrical activity is absolutely critical for any function of the brain, their main goal was to develop tissue culture conditions that physiologically supported such activity.
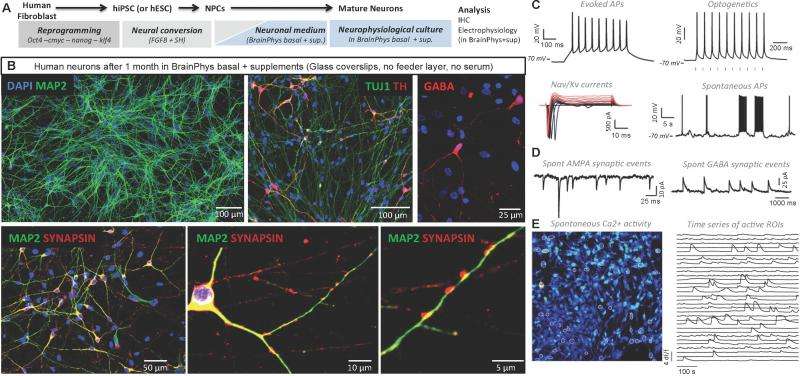
"Developing a new medium was not a simple task," Bardy recounts, "yet we could not ignore the fact that we were culturing our neurons in media that impaired their functions so dramatically. For some time we thought there might have been one component in the classic DMEM or Neurobasal media that was silencing the neuronal activity, the challenge being that these media comprise more than 50 components with different concentrations. The number of possible combinations was huge."
As a first attempt at identifying which components were critical for survival and neuronal activity, the scientists removed blocks of components – for example, all amino acids. Bardy says this was helpful, since it made clear that the problem was not just with one single component. "We managed to narrow down the list of possible problems, at which point we tried making intelligible guesses based on the literature as to what the key neuroactive components were. We also adjusted the concentrations of some of the components based on the compositions of natural human cerebrospinal fluid; we had to try several concentrations and a few different versions of the medium before finally arriving at BrainPhys, a medium that performs as well as aCSF in supporting neuronal electrical activity while also being able to support long-term survival of in vitro neuronal cultures."
A significant benefit is that over time, the enhancement of neuronal activity in the new neuronal medium was not detrimental to cell survival. Rather, the scientists found that long-term exposure to the BrainPhys medium significantly enhanced the synaptic function of human neurons. These functional improvements were accompanied by a significant increase in ARC protein, which is known to play a critical role in synaptic strength and memory consolidation.
Bardy notes that one of the main advantages of working with human iPSC technologies instead of animal models is having an experimental model that is closer to human – but it comes at the expense of losing the in vivo dimension of tissue. "As cell biologists, we have to compensate for this fact by pushing towards new tissue experimental models that will allow us to study tissue in vitro that better mimic the organization and functions of the same tissue in vivo. Beyond the medium in which cells are cultured," he explains, "many improvements should be addressed – for example, the physical substrate on which the neurons grow, or the oxygen levels in the incubator. BrainPhys helps us to study neuronal culture from a more accurate physiological perspective and should open the door to new, more rigorous studies looking specifically at neuronal and synaptic functions. This study also points out the need for cell biologists to rethink some of the tissue culture conditions that we have been using for decades without question. As researchers we should aim to constantly improve the accuracy and relevance of experimental models – not only in neuroscience research but also in other medical applications such as oncology, cardiology, and autoimmune disease." After all, Bardy tells Medical Xpress, since the emergence of human iPSC technologies, the efforts invested in modeling human neurological diseases in vitro have grown exponentially.
More information: Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro, Proceedings of the National Academy of Sciences (2015) published online before print, doi:10.1073/pnas.1504393112
© 2015 Medical Xpress