Dramatic remissions seen in immunotherapy trial of blood cancer patients

Twenty-seven out of 29 patients with an advanced blood cancer who received an experimental, "living" immunotherapy as part of a clinical trial experienced sustained remissions, according to preliminary results of the ongoing study at Fred Hutchinson Cancer Research Center.
Some of the patients in the trial, which began in 2013, were originally not expected to survive for more than a few months because their disease had previously relapsed or was resistant to other treatments, said Dr. Stanley Riddell, an immunotherapy researcher and oncologist Fred Hutch. Today, there is no sign of disease.
He shared the results on Sunday as part of an update on new adoptive T-cell therapy strategies for cancer at the annual meeting of the American Association for the Advancement of Science in Washington, D.C.
Riddell, who has studied how to empower the immune system to effectively treat human disease for more than 25 years, said that progress now being made, underscored by these latest results, is finally making immunotherapy "a pillar of cancer therapy."
But, he cautioned, "Much like chemotherapy and radiotherapy, it's not going to be a save-all." Some patients may require other treatments.
The trial is designed to test the safety of the latest iteration of an experimental immunotherapy in which a patient's own T cells are reprogrammed to eliminate his or her cancer. The reprogramming involves genetically engineering the T cells with synthetic molecules called chimeric antigen receptors, or CARs, that enable them to target and destroy tumor cells bearing a particular target. Trial participants include patients with acute lymphoblastic leukemia, non-Hodgkin lymphoma and chronic lymphocytic leukemia.
Because T cells can continue to multiply once infused into patients, the therapy does not have to be administered repeatedly, as is the case with chemotherapies that are eventually broken down by and eliminated from the body. And by introducing the CARs into two specific subsets of T cells—an approach pioneered at Fred Hutch—the researchers have achieved more potent and longer-lasting immune responses against tumors.
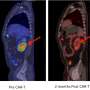
In one arm of the study led by Riddell's colleagues Drs. David Maloney and Cameron Turtle, 27 of 29 patients with acute lymphoblastic leukemia showed no trace of cancer in their bone marrow following their infusions. Nineteen of 30 non-Hodgkin lymphoma patients experienced partial or complete responses. In some patients, pounds of cancer were eliminated after a single dose of the engineered T cells—Riddell showed examples of patients whose tumors disappeared from imaging scans within weeks of the infusion.
The team has submitted a manuscript for journal publication describing their results with the first group of ALL patients.
T cells are white blood cells that can detect foreign or abnormal cells, including cancerous ones, and initiate a process that targets those abnormal cells for attack. But even when triggered, the natural immune response to a tumor is often neither strong nor persistent enough to overcome cancer cells. The T cells can become exhausted before all of the cancer is eliminated, and tumors can use a variety of techniques to evade them, including by usurping the normal checks and balances our immune system relies on to prevent overreactions. Engineering patients' T cells with CARs is one method researchers are testing to give the immune system the upper hand against the disease.
Riddell and his colleagues are constantly refining their process. They recently revised their CAR T-cell therapy protocols to try and make the approach more effective and reduce negative side effects, which can be severe and include neurological symptoms and "cytokine release syndrome," with fevers and drops in blood pressure. For example, among the ALL patients, the team found that giving the lowest doses of T cells to the patients with the highest tumor burdens reduced the risks of serious side effects. Before this risk-adapted dosing was implemented, seven patients with high tumor burdens required care in the intensive care unit for serious cytokine release syndrome; after incorporating the new dosing regimen, no high-burden patients needed ICU care.
Meanwhile, scientists in Riddell's lab and other labs at Fred Hutch are already developing the next generation of engineered T cells, which are expected to be safer and easier to design.
Hutch teams, as well as those at other centers, are also working to extend the successes seen so far in B-cell cancers to other common tumors, such as certain breast and lung cancers. While there are distinct challenges associated with targeting those types of disease compared to blood cancers, Riddell said he was optimistic that it would be possible to safely apply immunotherapies more broadly, so more patients can ultimately benefit.
The trial is funded by Juno Therapeutics, which was initially formed on technology from researchers at Fred Hutch, Memorial Sloan-Kettering Cancer Center and Seattle Children's Research Institute to commercialize promising immunotherapies.