Team reports a new mouse model to study how the EWS-FLI1 gene causes bone cancer
There exist several oncogenes that drive cancer. In many cases, however, the oncogenes themselves are not sufficient and must be complemented with other mutations before cancer develops. Researchers at the Center for iPS Cell Research and Application, Kyoto University, use cell reprogramming technology to revert cancer cells to a stem cell state. The researchers show that dysfunctional differentiation in conjunction with a specific oncogene could explain the cause of certain bone cancers.
Unlike normal cells, cancer cells proliferate uncontrollably, causing their spread throughout the body. This irregular proliferation is often attributed to mutant genes. Prof. Yasuhiro Yamada at the Center for iPS Cell Research and Application (CiRA), Kyoto University, is especially interested in one gene related to bone cancers. "One of our projects is the EWS-FLI1 gene", he said. This oncogene is considered necessary but not sufficient for several bone cancers, which suggests it must partner with other mutations to cause the cancer.
iPS cell technology has given cancer researchers a tool to reprogram cells and thus allows researchers to watch the cancer develop in real time. "We can modify the genes of iPS cells and then differentiate them to evaluate the importance of the mutation," says Prof. Yasuhiro Yamada, but added, "Most sarcomas [or cancer cells] are resistant to reprogramming." Fortunately for his lab, he discovered some sarcomas that are not.
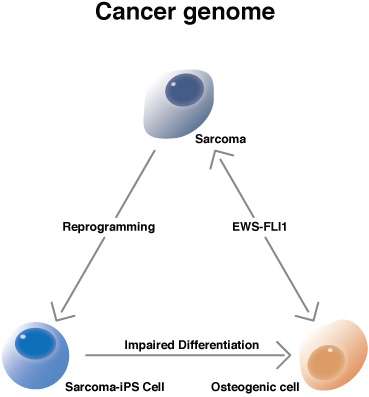
To identify the other mutations that cooperate with EWS-FLI1, his lab conducted an almost desperate experiment. They inserted into embryonic stem cells the EWS-FLI1 gene and inserted these cells into otherwise normal mice. An important feature of the cells is that the EWS-FLI1 gene is not expressed unless activated with an antibiotic, but the mice failed to grow tumors regardless. "This proves that other mutations are necessary," explained Yamada. However, when random mutations were added with the EWS-FLI1 gene, the mice grew tumors consistent of osteosarcomas, a type of bone cancer, when the EWS-FLI1 gene was activated. The researchers then attempted to reprogram the tumor cells into iPS cells (sarcoma-iPS cells), succeeding in two cases.
The acquisition of sarcoma-iPS cells allowed the scientists to observe how the additional mutations affect cell differentiation. In sarcoma-iPS cells in which the EWS-FLI1 gene was not activated, no tumors formed but aberrant differentiation was found. "Osteogenic cells [which go on to produce bone cells] did not develop properly," said Yamada. When the EWS-FLI1 gene was activated, the cells proceeded to form tumors. Yamada surmises that the unknown mutations affect the differentiation of osteogenic cells and that this mechanism is what makes the EWS-FLI1 gene oncogenic. He therefore proposes that the sarcoma-iPS cells could be valuable for drug discovery, as chemicals that correct the differentiation could prevent bone cancers from forming even in cases where the EWS-FLI1 gene is expressed. "This platform will be helpful to find small compounds for treatment," he said.