Breast cancer cells use newfound pathway to survive low oxygen levels in tumors

Researchers have identified a new signaling pathway that helps cancer cells cope with the lack of oxygen found inside tumors. These are the results of a study published in Nature Cell Biology on June 20, and led by researchers at NYU Langone Medical Center and its Laura and Isaac Perlmutter Cancer Center, Princess Margaret Cancer Center, the University of Toronto, Harvard Medical School and Oxford University.
Oxygen is important for the proper function of all human cells, but cancer cells thrive even when deprived of it. Rapid, abnormal cell growth seen in many solid tumors causes them to outgrow their blood supply and leave some cells with less oxygen. In the face of this "hypoxia," cancer cells change their gene expression to turn off all but the most vital oxygen-using processes.
"Our results, by yielding a new understanding of cancer cell response to hypoxia, hopefully will enable the design of future treatments that drive such cells into low-oxygen environments and then take away their ability to survive these conditions," says Benjamin Neel, MD, PhD, director of the Perlmutter Cancer Center.
In the new research, led by Robert Banh, a graduate student in Neel's lab, the investigators found that signals sent by the enzyme protein-tyrosine phosphatase 1B (PTP1B) work in a previously unknown way to shut down oxygen-using processes in breast cancer cells deprived of oxygen, thereby enhancing their survival.
Diabetes to Cancer to Moyamoya Disease
Neel and colleagues first identified the gene for protein-tyrosine phosphatase 1B (PTP1B) as part of search for molecules that suppress tumor growth in the early 1990s. PTP1B is the hallmark member of a group of enzymes that take a phosphate group away from biomolecules to turn processes like cell growth on or off.
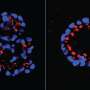
Neel and colleagues, as well as the group of Michel Tremblay at McGill University, found in earlier studies of mice that PTP1B function was required for the growth in certain cancers. These included breast cancers caused by the Her2 oncogene (HER2+ breast cancer cells), which is implicated in 20 percent of human breast cancers. In more recent work, Banh, Neel and colleagues found that human HER2+ breast cancer cells lacking PTP1B grew normally under standard culture conditions, but died much more rapidly in low oxygen.
Furthermore, researchers found that the three signaling pathways by which cancer cells were known to adapt to hypoxia worked fine in PTP1B-deficient HER2+ breast cancer cells. These included the well-known hypoxia-inducible factor pathway, which shifts the way cells use oxygen from oxidative phosphorylation in cellular "machines" called mitochondria to glycolysis, which does not require oxygen. Instead they found that other non-mitochondrial sources of oxygen consumption were not dialed down appropriately in PTP1B-deficient breast cancer cells.
The team further found that PTP1B controls the response of tumors in hypoxia by regulating the protein RNF213, which in in turn suppresses oxygen consumption by enzymes called α-ketoglutarate-dependent dioxygenases (α-KGDDs). These enzymes use oxygen, vitamin C (ascorbic acid) and iron to catalyze myriad reactions.
As the team began to fill in details concerning PTP1B pathway, they realized from the literature that RNF213 was also important in a rare condition called Moyamoya Disease, where patients experience abnormal blood vessel growth in the brain that can lead to blocked arteries and seizures. Conceivably, says Neel, Moyamoya disease symptoms could reflect an abnormal response to hypoxia in vascular cells, and his lab is working to understand the condition's molecular basis.
"We have seen many times in the cancer field that studies of rare syndromes can be being important in explaining mechanisms by which cells respond to stresses," says Neel. "We hope our new study will provide insights into Moyamoya disease that then feed back into our work in cancer biology."
More information: Nature Cell Biology, DOI: 10.1038/ncb3376