A docking site per calcium channel cluster
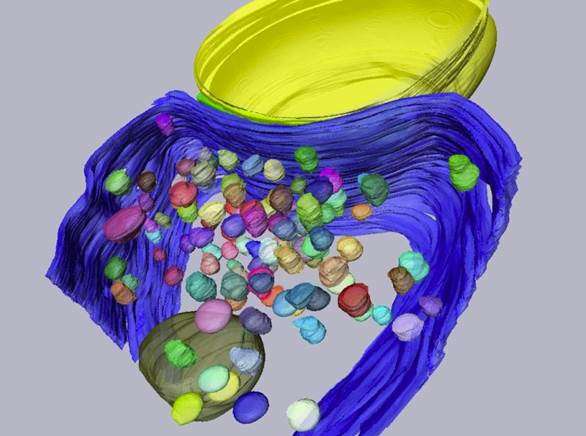
A study co-led by Ryuichi Shigemoto and Alain Marty concludes that a single docking site may use a single cluster of calcium channels, and that both the number of docking sites and the number of calcium clusters change in parallel with brain age. This establishes the first clear link between the morphology and function of docking sites. The study was published today in PNAS.
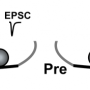
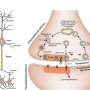
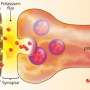
At a chemical synapse, signal transmission requires an elaborate sequence of events. It starts when an electrical signal, the action potential, reaches the synaptic terminal of the presynaptic neuron. This causes the voltage-gated calcium channel to open. Calcium ions rapidly stream into the presynaptic terminal and the calcium concentration in the presynaptic terminal rises. This allows synaptic vesicles filled with neurotransmitter to fuse with the plasma membrane and release the neurotransmitters into the synaptic cleft. Speed is essential in information transmission. Therefore, before the action potential even arrives at the presynaptic terminal, vesicles containing neurotransmitter line up in a fusion-ready state at docking sites in the presynaptic terminal. When the action potential reaches the presynaptic terminal, the vesicles can rapidly fuse and release the neurotransmitter. Functionally, docking sites limit the maximum number of vesicles that can be released at each action potential, determining the strength of the synapse. Until now, a clear link between the functional aspect of docking sites and their morphological aspect as sites where vesicles dock could not be established in the mammalian brain.
Shigemoto and colleagues used a high-resolution electron microscopy technique to look closely at the presynaptic terminal of a particular synapse in mice. They found that the number of functional docking sites matches the number of clusters of voltage-gated calcium channels in the presynaptic terminal. In addition, the number of docking sites and the number of calcium clusters change in parallel with brain age and synaptic size. This led the researchers to a major conclusion, as Shigemoto explains: "Based on our results, we suggest that for each docking site, there is a corresponding cluster of voltage-gated calcium channels. We propose a model in which each cluster of calcium channels is surrounded by enough free space to allow one synaptic vesicle to fuse in any direction."
More information: Takafumi Miki et al, Numbers of presynaptic Cachannel clusters match those of functionally defined vesicular docking sites in single central synapses, Proceedings of the National Academy of Sciences (2017). DOI: 10.1073/pnas.1704470114