Patching up a broken heart
It is almost impossible for an injured heart to fully mend itself. Within minutes of being deprived of oxygen – as happens during a heart attack when arteries to the heart are blocked – the heart's muscle cells start to die. Sanjay Sinha wants to mend these hearts so that they work again.
When the body's repair system kicks in, in an attempt to remove the dead heart cells, a thick layer of scar tissue begins to form. While this damage limitation process is vital to keep the heart pumping and the blood moving, the patient's problems have really only just begun.
Cardiac scar tissue is different to the rest of the heart. It doesn't contract or pump because it doesn't contain any new heart muscle cells. Those that are lost at the time of the heart attack never come back. This loss of function weakens the heart and, depending on the size of the damaged area, affects both the patient's quality of life and lifespan.
"In many patients, not only is their heart left much weaker than normal but they are unable to increase the amount of blood pumped around the body when needed during exercise," explains Dr Sanjay Sinha. "I've just walked up a flight of stairs… it's something I take for granted but many patients who've survived heart attacks struggle to do even basic things, like getting dressed. While there are treatments that improve the symptoms of heart failure, and some even improve survival to a limited extent, none of them tackles the underlying cause – the loss of up to a billion heart cells."
The numbers are stark. "Half a million people have heart failure in the UK. Almost half of them will not be alive in five years because of the damage to their heart. At present, the only way to really improve their heart function is to give them a heart transplant. There are only 200 heart transplants a year in the UK – it's a drop in the ocean when many thousands need them."
Sinha wants to mend these hearts so that they work again. "Not just by a few percent improvement but by a hundred percent."
He leads a team of stem cell biologists in the Cambridge Stem Cell Institute. Over the past five years, with funding from the British Heart Foundation, they have been working with materials scientists Professors Ruth Cameron and Serena Best and biochemist Professor Richard Farndale on an innovative technique for growing heart patches in the laboratory – with the aim of using these to repair weakened cardiac tissue.
"In the past, people have tried injecting cardiomyocytes into damaged hearts in animal models and shown that they can restore some of the muscle that's been lost," says Sinha. "But even in the best possible hands, ninety percent of the cells you inject are lost because of the hostile environment."
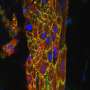
Instead, the Cambridge researchers are building tiny beating pieces of heart tissue in Petri dishes. The innovation that makes this possible is a scaffold. "The idea is to make a home for heart cells that really suits them to the ground. So they can survive and thrive and function."
The scaffold is made of collagen – a highly abundant protein in the animal kingdom. Best and Cameron are experts at creating complex collagen-based structures for a variety of cell types – bone marrow, breast cancer, musculoskeletal – both as implants and as model systems to test new therapeutics.
"The technology we've developed for culturing cells is exciting because it is adaptable to a huge range of applications – almost any situation where you're trying to regenerate new tissue," explains Best.
Best and Cameron use 'ice-templating' to build the scaffold. They freeze a solution of collagen, water and certain biological molecules. When the water crystals form, they push the other molecules to their boundaries. So, when the crystals are vapourised (by dropping the pressure to low levels), what's left is a complex three-dimensional warren.
"We have immense control over this structure," adds Cameron. "We can vary the pore structure to make cells align in certain orientations and control the ratios of cell types. We are building communities of millions of cells in an environment that resembles the heart."
Cardiomyocytes fare better when they are surrounded by other cell types and have something to hold on to. They use proteins on their surface called integrins to touch, stick to and communicate with their environment. Farndale has perfected a 'toolkit' that pinpoints exactly which parts of collagen the integrins bind best; he then makes matching peptide fragments to 'decorate' the collagen scaffold. This gives cells a foothold in the scaffold and encourages different cell types to move in and populate the structure.
"We don't just want a cardiac scaffold – we want it to have blood vessels and the same mechanical properties as the heart," explains Sinha. "If it's going to contract and function efficiently, it needs a really good blood supply. And the whole three-dimensional structure must be strong enough to survive the hostile environment of a damaged heart."
Meanwhile, Sinha's team pioneered the production of the different cell types needed for the patch. Their starting material is human embryonic stem cells, but they have also taken adult human cells and 'reset' their dev
elopmental clock. "In theory this means we can take a patient's own cells and make patches that are identical to their own tissue. That said, millions of people are going to need this sort of therapy and so our focus at the moment is on coming up with a system where a small number of patches might be available 'off the shelf', with patients receiving the nearest match.
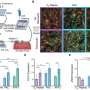
The team is completing tests on the ideal combination of scaffold structure, peptide decoration and mix of cells to create a beating vascularised tissue. Next, the researchers will work with Dr Thomas Krieg in the Department of Medicine to graft the tissue into a rat heart. Their aim is to show that the patch makes vascular connections, integrates mechanically and electrically with heart muscle, and contracts in synchrony with the rest of the heart. Once they've accomplished this, they will scale up the size of the patches for future use in people.
"It's exciting," says Sinha. "We are recreating a tissue that has all the components we see in an organ, where the cells start talking together in mysterious and wonderful ways, and they start to work together as they do in the body. Our vision is that this technology will bring hope to the millions of patients worldwide who are suffering from heart failure, and allow them to lead a normal life again."