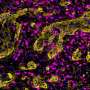
Researchers continue to seek strategy for starving brain tumors
In an effort to starve brain cancer cells and put the brakes on tumor development, University of North Carolina Lineberger Comprehensive Cancer Center researchers blocked the main pathway that brain tumor cells use to convert sugar into energy. They hoped this would starve tumor cells and slow their growth. To their surprise, however, the strategy actually accelerated growth in laboratory models of medulloblastoma.
Published in the journal Cancer Research, the study was part of a series of attempts by UNC Lineberger researchers to shut down the energy production machinery in medulloblastoma, the most common malignant brain tumor in children. The findings may help researchers identify a suitable therapeutic target within the sugar metabolism pathway, and provide clues to a scientific mystery surrounding the confounding way that some cancer cells get energy from sugar.
"Our goal is to continue to find out what's helping cancer cells to grow, and to try to stop it," said UNC Lineberger's Timothy R. Gershon, MD, PhD, an associate professor in the UNC School of Medicine Department of Neurology. "We're going to keep taking apart this energy production pathway in cancer cells—to conduct a molecular dissection to try to find the part that makes the cancer cells go."
Gershon said previous studies have shown that cancer cells rely on a process called "aerobic glycolysis," in which oxygen is present, but cells do not use it to get the maximum amount of energy from sugar. Cells using aerobic glycolysis need to use more sugar to get the same amount of energy, Gershon said.
"This is a question that scientists first started asking in the 1920s, and we're still trying to answer it," Gershon said. "Why would cancer cells use aerobic glycolysis?"
Glycolysis produces less energy overall, and creates a byproduct known as lactic acid. Gershon and his collaborators previously showed that normal cells that replicate in the growing brain rely on aerobic glycolysis.
"While most cells only use glycolysis when oxygen is not available, cancer cells use glycolysis all of the time, even under oxygen-rich conditions," he said. "No one knows why, but many people have hoped that blocking this form of glycolysis would be a way to treat cancer."
Researchers in Gershon's lab have blocked different molecular mechanisms that cells use during glycolysis to prevent them from producing energy. Previously, they found deleting a gene involved in glycolysis, Hexokinase 2, reduced brain tumor growth in preclinical models. However, Gershon said that gene would be hard to disrupt in people because it's similar to Hexokinase 1, which is vital for human survival. So, they searched for another target.
"We looked at other genes in the glycolysis pathway to see if they might be better targets for inhibitors, and to understand more about how the pathway supports cancer growth," Gershon said.
In the new study, they deleted a gene that codes for a molecule called pyruvate kinase. However, deleting this gene actually spurred cancer growth in laboratory models.
"This paper shows that some steps in glycolysis increase tumor growth, and other steps decrease tumor growth," Gershon said. "We do not know why, but it clearly makes a difference which step is targeted."
Together, the two studies show that the way cancer cells metabolize glucose significantly affects their proliferative behavior, the researchers reported. The most recent study points to actions upstream of the pyruvate kinase step as key to preventing cancer growth.
In addition to guiding future efforts to find a therapeutic target that could block cancer cells' energy production, the researchers may have identified a possible clue as to why cancer cells use this particular energy production pathway. They traced the path of sugar through the cell, and narrowed down the possibilities for where the glucose goes. Based on their findings, they speculate that medulloblastoma cells utilize the glucose to make proteins, which go into building more cells, rather than supplying energy.