First-ever look at potentially deadly metabolic disorder that strikes infants
You may have never heard of congenital disorder of glycosylation, but parents whose children are born with forms of this rare – and underreported – metabolic disorder know all too well the dangers they pose, including developmental delay, failure to thrive, stroke-like symptoms, seizures and cerebellar dysfunction.
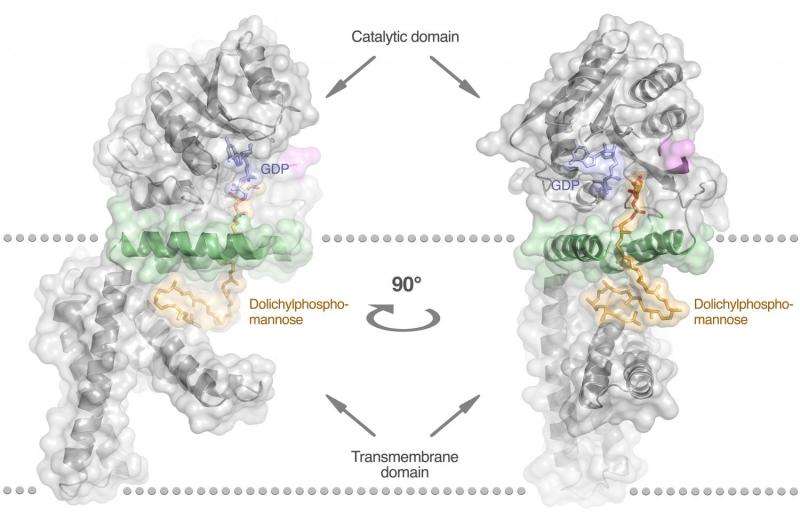
Often serious, and sometimes fatal, CDG diseases are hereditary disorders that affect a complex metabolic process, known as glycosylation, which is critical for the development of organs. Reporting in the journal Nature Communications, researchers from KTH Royal Institute of Technology in Stockholm have demonstrated for the first time the way in which certain types of CDG diseases arise from the shortage of a fundamental building block for proteins, the enzyme dolichylphosphomannose, which is known as DPMS.
Glycosylation produces sugar chains that are attached to the surface of proteins. The majority of proteins produced in the human body, about two thirds, undergo the maturation process to become glycoproteins. A glycoprotein obtains its final shape and function once the sugar chains are in place.
Although there is yet no cure for CDG, the researchers in Sweden have given us an unprecedented look at the inner workings of DPMS. KTH Biotechnology Professor Christina Divne says the team hope that their work will lead to improved chances of predicting and treating CDG.
The article in Nature Communication details how the team determined the three-dimensional atomic structure of DPMS, using a technically-advanced and time-consuming method.
"From the very 3-D image of the enzyme's appearance, we have managed to determine how it functions; and by that explain why disease-causing variants of DPMS lack the ability to produce the necessary building block, and therefore cause disease," Divne says.
The study also sheds new light on how aberrant DPMS causes severe psychomotor developmental delays, microcephaly, epileptic seizures, and sometimes body malformations. A connection has also been found to certain forms of cancer.
First identified in 1980, CDG cases have been documented in limited numbers. Divne says that many cases are likely unrecorded since a correct diagnosis requires that the sequences for known disease genes be determined – a step that is not regularly performed. Diagnosis also requires knowledge of relevant genetic variants.
"CDG diseases are often misdiagnosed and mistaken for other disease states," she says. "The symptoms resemble those of many other neurometabolic diseases and physicians do not always know what they are looking for."
She says that genetic variants that cause CDG disease most likely occur at a considerably higher frequency than what can be observed in a population. Most CDG diseases cause severe disabilities, and probably lead to premature death of the fetus and spontaneous abortion. "A spontaneously aborted fetus rarely ends up at a hospital to be gene tested," she notes.
About 120 different glycosylation disorders are currently known, a number that has increased threefold in 10 years, and there are probably many more to be discovered, she says. In a Canadian study on patients examined for unexplained developmental retardation, more than 10 percent could be traced to genetic defects in glycosylation processes.
More information: Rosaria Gandini et al. Structural basis for dolichylphosphate mannose biosynthesis, Nature Communications (2017). DOI: 10.1038/s41467-017-00187-2