Study of nervous system cells can help to understand degenerative diseases
The results of a new study show that many of the genes expressed by microglia differ between humans and mice, which are frequently used as animal models in research on Alzheimer's disease and other neurodegenerative disorders.
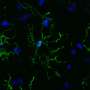
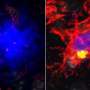
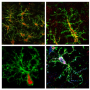
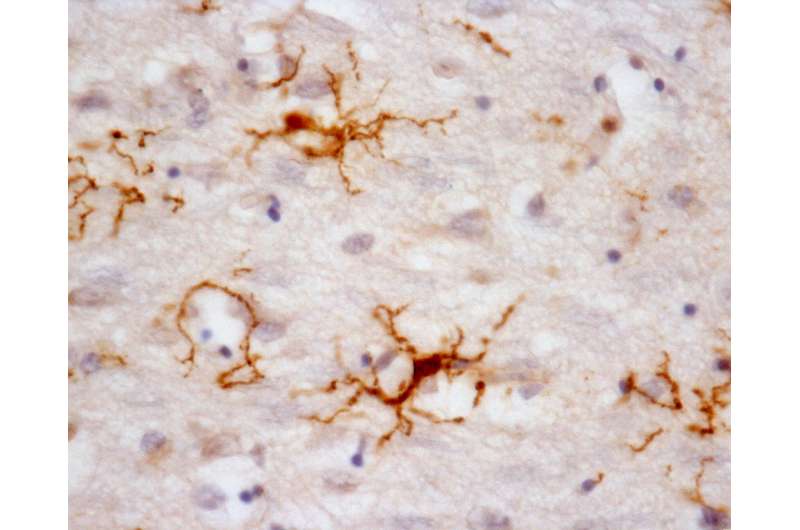
Microglia are the primary immune cells of the central nervous system, with functions similar to those of white blood cells, especially in actively defending the brain and spinal cord. The collaborative research by Brazilian and Dutch scientists on the main genes expressed by human microglia were published in Nature Neuroscience. It also shows that human microglial cells age differently from those of mice. The research was supported by the São Paulo Research Foundation (FAPESP).
"The findings are important for studies of the gene expression profiles of normal microglia during human aging. They can serve as a basis for comparisons designed to detect changes in microglia as part of several neurodegenerative diseases," said Suely Nagahashi Marie, who heads the Molecular & Cellular Biology Laboratory at the University of São Paulo's Medical School (FM-USP).
Microglia terminate on neurons and inspect their surroundings in search of external agents to fight off, dead synapses to remove, and neurons that are dying and require elimination. When they detect a problem, they move swiftly to phagocytize the inflammation-causing agent. Given the role of microglia in the central nervous system's immune responses, these cells are also a focus for research on neurodegenerative diseases such as Parkinson's disease, amyotrophic lateral sclerosis (ALS) and Alzheimer's.
"In order to understand the role of microglia in neurodegenerative diseases, we first have to know which genes in human microglia are expressed at the highest levels in a healthy nervous system," said biomedical scientist Thais Fernanda de Almeida Galatro, another author of the research project. "This has never been investigated. Our idea was to establish a gene expression profile of human microglia."
Galatro learned a technique for sampling cortical microglia from the team working under Bart Eggen, a professor in Gronigen's Department of Neuroscience. The collaboration included a visit by a member of Eggen's team to the laboratory headed by Nagahashi Marie. The cortical samples used in the research were collected from autopsies performed in the Netherlands and by São Paulo City's Death Certification Service (SVOC), which donated them to the Human Brain Bank of FM-USP's Cerebral Aging Study Group.
Thirty-nine of 81 samples were selected from individuals without a history of cerebral pathology. "This is so we could be sure the microglia used in the study were from healthy brains," Galatro said. The selected samples were from men and women aged between 34 and 102 years. This wide age range was deliberately chosen, because the researchers wanted to understand how aging affects microglial gene expression.
The microglia obtained from the cortical samples were submitted to transcriptome (RNA) sequencing. "Next-generation sequencing is used to assess the level of expression of all genes encoded in the sample analyzed. In our study, we used a technique called ribosomal RNA depletion. Ribosomal RNA accounts for about 80 percent of total microglial RNA," Nagahashi Marie said.
This approach enabled the researchers to perform an accurate count of protein-encoding messenger RNAs. They detected between 17,000 and 19,000 human microglial genes. "We compared "these large sets of genes between two groups—microglia in isolation versus total brain tissue," she explained.
Microglia represent a specific cellular compartment of brain tissue. In the comparison between microglia and total brain tissue, the 17,000 to 19,000 genes were ranked according to their abundance in each of the compartments. As a result, the researchers were able to identify the 1,297 genes that were most abundantly expressed in human microglia.
"This group of genes was considered the molecular signature of human microglia," Nagahashi Marie said.
Having isolated the 1,297 most highly expressed genes in human microglia, the researchers compared these genes with murine microglial genes. Most research on microglia uses a murine model.
"We found that the most expressed microglial genes in humans and mice are similar to those most expressed in general terms," Galatro said. In both humans and mice, these genes relate mainly to the functions of motility (microglial cells move through nerve tissue) and defense (phagocytosis of pathological agents).
However, a small number of the most highly expressed human microglial genes are unique to our species and are not found in mice. "We discovered that these few exclusively human genes play an important role in the immune response. In other words, they're important to the host organism's defense against infections," Galatro said.
Comparison with primates
According to Nagahashi Marie, the main outcome of the research project was the demonstration of this key difference despite the large overlap between human and murine microglial genes. "The implication is that the results of experiments in murine models of neurodegeneration, including models of Alzheimer's, must be interpreted with caution," she said. "Our results for the expression of human microglia during the aging process differ from those described hitherto for the murine model of aging."
The next step for the group is to analyze the functions of the genes identified in order to discover what roles they play in the normal physiology of the brain and to understand the changes that take place in neurodegenerative diseases. Having compared human and murine genes, the researchers are naturally interested in comparisons with the microglia of animals that are closer to humans in evolutionary terms.
"The sequencing of primate microglia is underway in our lab," Nagahashi Marie said. "When we have the primate transcriptome, we'll be able to compare the evolution of human microglial gene expression with that of microglia in other primates, mice and zebrafish."
The researchers have also sequenced microglia isolated from seven brain regions in autopsied individuals without cognitive dysfunction, and they are analyzing the samples using bioinformatics. "The new study will enable us to see whether there are differences between microglia in different parts of the brain," she said. "Our hypothesis is that there are, and that microglia are relevant to the pathology of neurodegenerative diseases. We're therefore including in our analysis the brain regions most compromised in the progression of Alzheimer's."
More information: Thais F Galatro et al. Transcriptomic analysis of purified human cortical microglia reveals age-associated changes, Nature Neuroscience (2017). DOI: 10.1038/nn.4597