Mutant zebrafish help explain the cause of a rare muscle-degenerating disorder
An immobile mutant zebrafish first described by scientists more than 20 years ago turns out to have defects in the same gene as people with a rare muscle-degenerating disorder called nemaline myopathy, an A*STAR study has found.
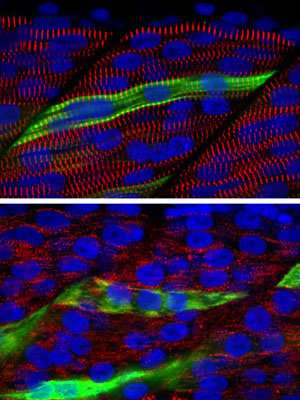
The study points to a central role of the faulty gene, known as myo18b, in the assembly of sarcomeres, the force-generating component of the muscle cell. It also helps explain the poor motor function seen in patients who have mutations in this gene—an insight that mouse models could not provide, because mouse embryos that lack myo18b die before their skeletal muscle has developed.
As Philip Ingham, head of the Developmental and Biomedical Genetics Laboratory at the A*STAR Institute of Molecular and Cell Biology, points out: "The zebrafish mutant provides the only animal model for studying the effects of the myo18b mutation on skeletal muscle."
Ingham worked with postdoctoral fellows in Singapore and England to characterize a particular strain of mutant zebrafish first identified by German researchers in 1996. At the time, the scientists were looking for gene mutations that affect zebrafish motility, and they found one that meant the developing fish embryos were without fast-twitch muscle fibers, bundles of long and slender cells that are needed for bursts of rapid movement.
They named the mutated gene frozen. However, they had no idea where the gene was in the zebrafish genome or what kind of protein it encoded.
Ingham and his team have now filled in those missing details. They used a series of genetic markers and fish breeding experiments to narrow down the location of frozen to a relatively short stretch of around 1.4 million DNA letters long on chromosome 10. The researchers then compared this small region with matching DNA from other fish species, including tilapia and pufferfish.
They found that what had been called frozen is actually the zebrafish version of a known gene, myo18b, which encodes a poorly characterized member of the myosin motor protein family. In the zebrafish, the protein-coding portion of the myo18b gene was about 50 per cent identical to its human counterpart, which has been linked to rare genetic muscle disorders.
Detailed analyses of these myo18b-deficient fish provide the first definitive evidence that this motor protein is needed for proper skeletal muscle development. Ingham says the fish can now serve "as a model for better understanding the role of MYO18B in the assembly of the sarcomere," and he hopes they can help scientists find ways to treat myopathy disorders in people.
More information: Ritika Gurung et al. A Zebrafish Model for a Human Myopathy Associated with Mutation of the Unconventional Myosin MYO18B, Genetics (2016). DOI: 10.1534/genetics.116.192864