Study shows routine genomic surveillance of MRSA can detect unsuspected outbreaks
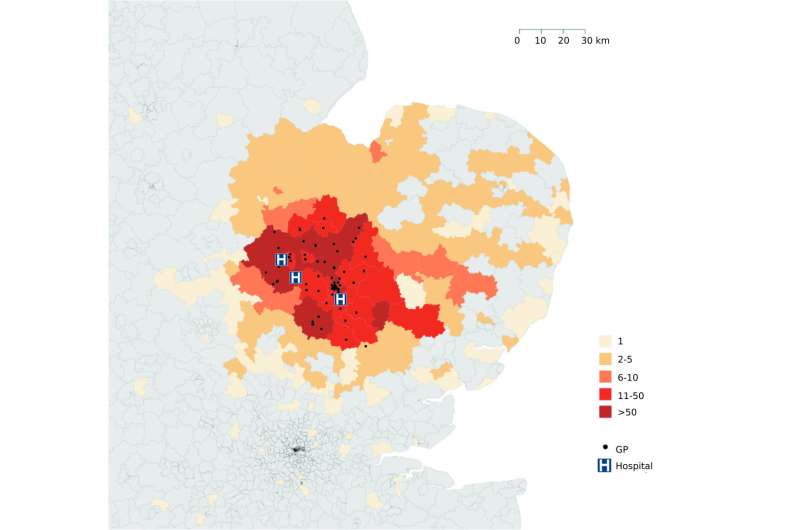
Genomic surveillance has revealed the first complete picture of MRSA spread across the east of England. Researchers from the Wellcome Trust Sanger Institute and the London School of Hygiene & Tropical Medicine tracked MRSA-positive people and were able to describe the complete picture of MRSA transmission within and between hospitals, and in GPs surgeries and communities.
The study, reported today (25th October) in Science Translational Medicine, has important implications for infection control. It shows that sequencing of MRSA (methicillin-resistant Staphylococcus aureus) whenever someone is detected with the strain, and combining this with information on when and where transmission may have occurred, could link cases and detect outbreaks much sooner. This would help to prevent further transmission and reduce the number of people involved.
MRSA is a strain of a bacterium that is resistant to many antibiotics and is a leading cause of infections associated with hospitals and healthcare. Bloodstream infection caused by MRSA is difficult to treat and results in the death of up to a fifth of patients affected. Even when the infection is treated, MRSA infections double the average length of hospital stays and increase healthcare costs. The World Health Organization recently classified MRSA as high priority on their priority pathogens list for Research and Development of new drugs.
Patients may be screened for MRSA after they are admitted to hospital, where infection control measures are used to monitor and try to reduce the spread of MRSA. When new MRSA carriers are detected, this is investigated to confirm whether an outbreak is underway, looking for links between patients in time and place. However, this current approach is not very sensitive, especially given the speed with which patients can move around different parts of the hospital or even between hospitals during their care. This approach can also miss MRSA transmission between people in hospitals and the community, and outbreaks in family or community groups can be difficult to detect.
The study reported by Dr Francesc Coll and colleagues tracked everyone who had an MRSA-positive sample in the east of England over one year. They sequenced the DNA of at least one MRSA strain from 1465 people, based on routine samples submitted to a microbiology laboratory that served 3 hospitals and 75 GP surgeries. They detected 173 different outbreaks, most of which had not been previously spotted, and discovered MRSA outbreaks in hospitals, in the community, GP surgeries, homes and in between these places.
Dr Julian Parkhill, an author on the paper from the Wellcome Trust Sanger Institute, said: "Using whole genome sequencing we have been able to see the full picture of MRSA transmission within hospitals and the community for the first time. We found that sequencing MRSA from all affected patients detected many more outbreaks than standard infection control approaches. This method could also exclude suspected outbreaks, allowing health authorities to rationalise resources."
Dr Jonathan Pearce, head of infections and immunity at the MRC, said: "Antibiotic resistance poses a global challenge to healthcare. To tackle it we need to prevent infections, preserve existing antibiotics and promote the development of new therapies and interventions. This study sheds light on MRSA transmission within and between hospitals and the community, which could help strengthen infection prevention and control measures."
The technology for sequencing MRSA strains has advanced rapidly, allowing the strain information to be obtained much faster than was previously possible, and more economically. A follow-up study will start next year, during which the researchers will sequence MRSA strains from all new cases and share strain and tracking information in real time with infection control workers. This will aim to help detect and exclude outbreaks, allowing targeted and effective interventions.
Professor Sharon Peacock, the study lead at the Wellcome Trust Sanger Institute and the London School of Hygiene & Tropical Medicine, said: "Our study has shown that sequencing all MRSA samples as soon as they are isolated can rapidly pinpoint where MRSA transmission is occurring. If implemented in clinical practice this would provide numerous opportunities to catch outbreaks early and target these to bring them to a close, for example by decolonising carriers and implementing barrier nursing. We have the technology in place to do this and it could have a really positive impact on public health and patient outcomes."
More information: F. Coll el al., "Longitudinal genomic surveillance of MRSA in the UK reveals transmission patterns in hospitals and the community," Science Translational Medicine (2017). stm.sciencemag.org/lookup/doi/ … scitranslmed.aak9745