Researchers find infectious prions in Creutzfeldt-Jakob disease patient skin
Creutzfeldt-Jakob disease (CJD)—the human equivalent of mad cow disease—is caused by rogue, misfolded protein aggregates termed prions, which are infectious and cause fatal damages in the patient's brain. CJD patients develop signature microscopic sponge-like holes in their brains. The initial signs of CJD include memory loss, behavior changes, movement disorder, and vision problems, which usually rapidly progress to death. According to the National Institutes of Health (NIH), 90 percent of CJD patients die within one year of onset, and hundreds of Americans are diagnosed annually. There is no available treatment or cure.
There are numerous types of prion diseases in humans, and CJD is the most common. About 90 percent of CJD cases have a sporadic origin. Prion infectivity is highly concentrated in CJD patient brain tissue. Inter-personal CJD transmission has occurred after patients were exposed to surgical tools previously contaminated by CJD brain tissues.
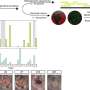
But in a Science Translational Medicine study published today, Case Western Reserve University School of Medicine researchers found that CJD patients also harbor infectious prions in their skin, albeit at lower levels. In the study, the researchers collected skin samples from 38 patients with assistance from the National Prion Disease Pathology Surveillance Center at Case Western Reserve School of Medicine and measured their prion levels. Using a highly sensitive in vitro assay developed and conducted by Byron Caughey's group at the NIH, they detected prion protein aggregates in the skin samples from all of CJD patients. Prion levels were 1,000-100,000 times lower in the skin than in the brain, and only detectable by this extremely sensitive assay. The researchers further demonstrated that such skin prions are infectious, since they are capable of causing disease in humanized mouse models.
This unexpected finding raises a host of issues. "It is well known that CJD is transmissible via surgical or medical procedures involving prion-infected brain tissue. Our finding of infectious prions in skin is important since it not only raises concerns about the potential for disease transmission via common surgeries not involving the brain, but also suggests that skin biopsies and autopsies may enhance pre-mortem and post-mortem CJD diagnosis," said Wenquan Zou, Associate Professor of Pathology and Neurology and Associate Director of the National Prion Disease Pathology Surveillance Center at Case Western Reserve School of Medicine. Zou led the study involving a consortium of research groups and researchers across Case Western Reserve School of Medicine, University Hospitals Cleveland Medical Center, the NIH, and the People's Republic of China.
"The level of prion infectivity detected in CJD skin was surprisingly significant, but still much lower than that in CJD brains," cautioned Qingzhong Kong, Associate Professor of Pathology and Neurology at Case Western Reserve School of Medicine. "Prion transmission risk from surgical instruments contaminated by skin prions should be much lower than that of instruments contaminated by brain tissue." In the study, the Kong group assisted by the Zou group demonstrated that CJD patient skin is infectious using humanized transgenic mouse models.
Current diagnostic tools for CJD rely on brain tissue samples collected at either biopsy or autopsy, or cerebral spinal fluid obtained by spinal taps. The new study may lay the foundation for less invasive techniques. "Using the skin instead of brain tissue for post-mortem diagnosis could be particularly helpful in cultures that discourage brain autopsy, such as China and India. These countries have the largest populations with the greatest number of patients, but brain autopsy is often not performed," said Zou.
"Further investigation is necessary to determine whether extra precautions should be taken during non-neurosurgeries of CJD patients, especially when surgical instruments will be reused," said Zou. Case Western Reserve School of Medicine researchers plan to further evaluate the potential risk of skin prion transmission through non-neurosurgeries, primarily using mouse models.
More information: C.D. Orru el al., "Prion seeding activity and infectivity in skin samples from patients with sporadic Creutzfeldt-Jakob disease," Science Translational Medicine (2017). stm.sciencemag.org/lookup/doi/ … scitranslmed.aam7785