Researchers identify a crucial protein that commands a key communications hub determining cell growth
National University of Singapore biologists have identified a crucial protein that commands a key communications hub which determines cell growth.
Various proteins control key physiological functions when their activities are properly regulated. However, the same set of proteins could lead to undesirable outcomes if their amounts are at abnormal levels or their cell-to-cell communications are short-circuited. Two cell signalling pathways, the RAS/MEK and JNK pathways (named after distinct proteins that play crucial roles in controlling cell growth and cell death, respectively) are well-known to influence the way cells grow, divide or even commit suicide. Although various studies have shown that these two pathways can work in parallel or together to control cell fates, it is not clear how they influence or work with each other to promote cell proliferation, which can sometimes prime cells towards uncontrolled growth in cancers.
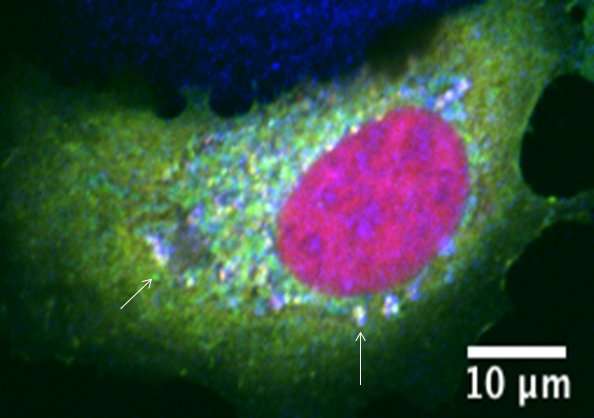
A team of researchers led by Prof Boon Chuan LOW from the Department of Biological Sciences, NUS has identified BPGAP1 as the key scaffold protein that is responsible for linking these two major signalling pathways together. This protein acts like a switch controlling the genetic programming for cell growth. When this protein is functioning properly, the two pathways can communicate with each other to maintain a balance in cell numbers. If it malfunctions, it could lead to uncontrolled growth of cells, causing cancer development. The team has also established the crucial steps involved in this precise activation mechanism at the cellular and molecular levels.
"The abnormal activation of certain proteins (epidermal growth factor receptor, RAS, MEK and JNK) are known to be associated with different ways of causing cancers. The identification of BPGAP1 represents a new one-stop hub that controls these activities. This opens the way for developing potential new therapeutics that could prevent tumour growth," said Prof Low, who is also a Senior Principal Investigator at the Mechanobiology Institute, NUS.
The group plans to further delineate the precise sites and structure of the interaction between BPGAP1 and the JNK and MEK proteins, so as to pinpoint the most desirable sites on the molecule for the development of anti-cancer therapeutics.
More information: T Jiang et al. BPGAP1 spatially integrates JNK/ERK signaling crosstalk in oncogenesis, Oncogene (2017). DOI: 10.1038/onc.2016.466