Researchers identify gene responsible for mesenchymal stem cells' stem-ness'

Many doctors, researchers and patients are eager to take advantage of the promise of stem cell therapies to heal damaged tissues and replace dysfunctional cells. Hundreds of ongoing clinical trials are currently delivering these therapies to patients worldwide.
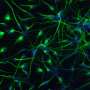
Mesenchymal stem cells (MSCs) are popular tools for these therapies because they can differentiate into a variety of mature cell types such as bone, fat and cartilage. They also support hematopoiesis—the formation of blood cells—and secrete beneficial factors that promote tissue repair. Unfortunately, scientists often struggle to predict how these cells will act in different environments in the body.
Researchers on the Florida campus of The Scripps Research Institute (TSRI) recently published a study in the journal Cell Death and Differentiation identifying factors crucial to MSC differentiation, providing insight into how these cells should be studied for clinical purposes.
Primary MSCs from bone marrow are delicate, and it´s difficult to keep them alive in a petri dish. For this reason, most scientists studying them use cell lines, cells that have been altered to live and replicate indefinitely. To create immortal MSC lines, scientists delete a gene called p53 required for the cells to undergo normal, programmed cell death, called apoptosis. The new study, conducted by researchers in the lab of TSRI Professor Donald Phinney, PhD, shows that the gene influences far more than just apoptosis; it is also a master regulator of the cells' ability to differentiate.
"Many publications have used immortalized cells as MSC surrogates, but they may not mean much if they don't have functionally accurate p53," says Siddaraju Boregowda, PhD, a TSRI research associate and one of the study's two lead authors.
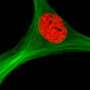
The researchers compared cells cultured from mice that did not express the p53 gene with cells that came from normal mice. They found that the level of active p53 was the master regulatory factor in determining how MSCs grow and differentiate. The study explains that the gene exerts its effects through its interactions with reactive oxygen species as well as two transcription factors: TWIST2 and PPARG.
When the researchers deleted p53 completely, the cells became immortal but quickly developed into bone. They lost their ability to promote hematopoiesis or become other types of cells, like fat. A low level of p53 induced TWIST2, which kept the MSCs in a stem state, rather than promoting any differentiation. A slightly higher level of p53 induced PPARG and reactive oxygen species, which led the cells to differentiate into fat cells, but not bone. At even higher levels of p53, the cells died.
A basal level of p53 in cells in the culture is required for them to act as an accurate model for cells in the body, explains Veena Krishnappa, PhD, the study's other lead author, previously a postdoctoral researcher in Phinney's lab.
In addition to suggesting that the dramatic effects of deleting p53 may make MSC cell lines an inappropriate surrogate to predict the cells' behavior in clinical applications, the study also suggests that inactivation of p53 may play multiple roles in the progression of bone cancers.
"P53 inactivation not only promotes sustained growth but also makes the cells insensitive to oxidative stress and short-circuits pathways that constrain cellular differentiation. Each of these changes likely contribute significantly to tumorigenesis and tumor progression," says Phinney. "Continuing to delineate the important role of MSCs in skeletal disease may open the door to devising new therapeutic interventions."
More information: Siddaraju V. Boregowda et al. Basal p53 expression is indispensable for mesenchymal stem cell integrity, Cell Death & Differentiation (2018). DOI: 10.1038/s41418-017-0004-4